Impact of sub-optimal HIV viral control on activated T cells
- PMID: 36723505
- PMCID: PMC7617099
- DOI: 10.1097/QAD.0000000000003488
Impact of sub-optimal HIV viral control on activated T cells
Abstract
Objective: HIV viral load (VL) monitoring is generally conducted 6-12 monthly in low- and middle-income countries, risking relatively prolonged periods of poor viral control. We explored the effects of different levels of loss of viral control on immune reconstitution and activation.
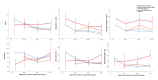
Design: Two hundred and eight participants starting protease inhibitor (PI)-based second-line therapy in the EARNEST trial (ISRCTN37737787) in Uganda and Zimbabwe were enrolled and CD38 + /HLA-DR + immunophenotyping performed (CD8-FITC/CD38-PE/CD3-PerCP/HLA-DR-APC; centrally gated) in real-time at 0, 12, 48, 96 and 144 weeks from randomization.
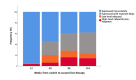
Methods: VL was assayed retrospectively on samples collected every 12-16 weeks and classified as continuous suppression (<40 copies/ml throughout); suppression with transient blips; low-level rebound (two or more consecutive VL >40, <5000 copies/ml); high-level rebound/nonresponse (two or more consecutive VL >5000 copies/ml).
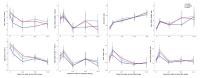
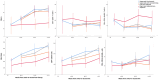
Results: Immunophenotype reconstitution varied between that defined by numbers of cells and that defined by cell percentages. Furthermore, VL dynamics were associated with substantial differences in expression of CD4 + and CD8 + cell activation markers, with only individuals with high-level rebound/nonresponse (>5000 copies/ml) experiencing significantly greater activation and impaired reconstitution. There was little difference between participants who suppressed consistently and who exhibited transient blips or even low-level rebound by 144 weeks ( P > 0.2 vs. suppressed consistently).
Conclusion: Detectable viral load below the threshold at which WHO guidelines recommend that treatment can be maintained without switching (1000 copies/ml) appear to have at most, small effects on reconstitution and activation, for patients taking a PI-based second-line regimen.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Figures
References
-
- Paton NI, Kityo C, Hoppe A, Reid A, Kambugu A, Lugemwa A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. The New England journal of medicine. 2014;371(3):234–247. - PubMed
-
- Paton NI, Bagenda L, van Oosterhout JJ, Bertagnolio S, Easterbrook PJ, Walker AS, et al. Nucleoside reverse-transcriptase inhibitor cross-resistance and outcomes from second-line antiretroviral therapy in the public health approach: an observational analysis within the randomised, open-label, EARNEST trial. The Lancet HIV. 2017;4(8):e341–e348. doi: 10.1016/S2352-3018(17)30065-6. - DOI - PMC - PubMed
-
- Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narváez AB, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

