Lack of Paxillin phosphorylation promotes single-cell migration in vivo
- PMID: 36723624
- PMCID: PMC9929932
- DOI: 10.1083/jcb.202206078
Lack of Paxillin phosphorylation promotes single-cell migration in vivo
Abstract
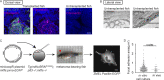
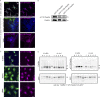
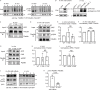
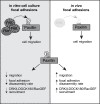
Focal adhesions are structures that physically link the cell to the extracellular matrix for cell migration. Although cell culture studies have provided a wealth of information regarding focal adhesion biology, it is critical to understand how focal adhesions are dynamically regulated in their native environment. We developed a zebrafish system to visualize focal adhesion structures during single-cell migration in vivo. We find that a key site of phosphoregulation (Y118) on Paxillin exhibits reduced phosphorylation in migrating cells in vivo compared to in vitro. Furthermore, expression of a non-phosphorylatable version of Y118-Paxillin increases focal adhesion disassembly and promotes cell migration in vivo, despite inhibiting cell migration in vitro. Using a mouse model, we further find that the upstream kinase, focal adhesion kinase, is downregulated in cells in vivo, and cells expressing non-phosphorylatable Y118-Paxillin exhibit increased activation of the CRKII-DOCK180/RacGEF pathway. Our findings provide significant new insight into the intrinsic regulation of focal adhesions in cells migrating in their native environment.
© 2023 Xue et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







Comment in
-
An in vivo phosphoregulation paradox for focal adhesions.J Cell Biol. 2023 Mar 6;222(3):e202301060. doi: 10.1083/jcb.202301060. Epub 2023 Feb 16. J Cell Biol. 2023. PMID: 36795454 Free PMC article.
References
-
- Balaban, N.Q., Schwarz U.S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L., and Geiger B.. 2001. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. 10.1038/35074532 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

