Long-term Efficacy of Satralizumab in AQP4-IgG-Seropositive Neuromyelitis Optica Spectrum Disorder From SAkuraSky and SAkuraStar
- PMID: 36724181
- PMCID: PMC9756307
- DOI: 10.1212/NXI.0000000000200071
Long-term Efficacy of Satralizumab in AQP4-IgG-Seropositive Neuromyelitis Optica Spectrum Disorder From SAkuraSky and SAkuraStar
Abstract
Background and objectives: Satralizumab, an interleukin 6 receptor inhibitor, reduced the risk of protocol-defined relapse (PDR) vs placebo in 2 independent, double-blind studies in patients with neuromyelitis optica spectrum disorder (NMOSD). We assessed the long-term efficacy of satralizumab in patients with aquaporin-4-immunoglobulin G (IgG)-seropositive (AQP4-IgG+) NMOSD.
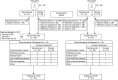
Methods: Following the double-blind periods of SAkuraSky (satralizumab + baseline immunosuppressive treatment [IST]) and SAkuraStar (satralizumab monotherapy), patients could enter the open-label extension (OLE, satralizumab 120 mg Q4W ± IST). This analysis included all AQP4-IgG+ patients who received ≥1 dose of satralizumab in the double-blind and/or OLE periods, from patients' first dose to the data cutoff (February 22, 2021). PDR in the OLE period was determined by the investigator without external adjudication. We evaluated time to first investigator-reported PDR (iPDR), severe iPDR (≥2 point increase in the Expanded Disability Status Scale [EDSS] score), and sustained EDSS worsening (EDSS score increase of ≥2, ≥1, or ≥0.5 points for patients with baseline scores of 0, 1-5, or ≥5.5, respectively, confirmed ≥24 weeks post-initial worsening), plus the annualized iPDR rate (ARR).
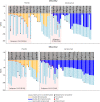
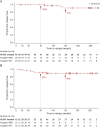
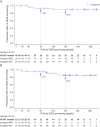
Results: Forty-six of 55 AQP4-IgG+ patients (84%) in SAkuraSky and 57/64 patients in SAkuraStar (89%) continued from the double-blind periods into the OLEs. In total, 111 AQP4-IgG+ patients received ≥1 dose of satralizumab in the double-blind and/or OLE periods and were included in these analyses (SAkuraSky: 49; SAkuraStar: 62). The median (range) duration of satralizumab exposure was 4.4 (0.1-7.0) years in SAkuraSky and 4.0 (0.1-6.0) years in SAkuraStar, with a combined 440.1 patient-years of treatment. Seventy-one of 111 patients (64%) received satralizumab for ≥192 weeks (3.7 years). At this time point, 71% (SAkuraSky) and 73% (SAkuraStar) of satralizumab-treated patients were free from iPDR, 91% (SAkuraSky) and 90% (SAkuraStar) were free from severe iPDR, and 90% (SAkuraSky) and 86% (SAkuraStar) had no sustained EDSS worsening. The overall adjusted ARR (95% CI) was 0.12 (0.08-0.18) in SAkuraSky and 0.08 (0.05-0.13) in SAkuraStar and remained stable over time.
Discussion: These long-term results from the OLE periods of the SAkura studies demonstrate the continued efficacy of satralizumab over more than 3.5 years of treatment. High proportions of patients remained free from relapse, severe relapse, or worsening disease, with a consistently low ARR.
Trial registration information: ClinicalTrials.gov registration numbers: NCT02028884 (SAkuraSky) and NCT02073279 (SAkuraStar).
Classification of evidence: This study provides Class II evidence that satralizumab reduces the risk of relapse in patients with AQP4-IgG+ NMOSD beyond the first 96 weeks of treatment.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
