NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8
- PMID: 36724221
- PMCID: PMC9891695
- DOI: 10.1126/sciadv.ade9585
NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8
Abstract
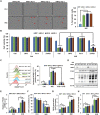
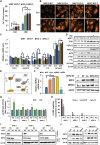
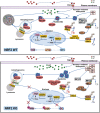
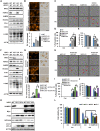
Enhancing the intracellular labile iron pool (LIP) represents a powerful, yet untapped strategy for driving ferroptotic death of cancer cells. Here, we show that NRF2 maintains iron homeostasis by controlling HERC2 (E3 ubiquitin ligase for NCOA4 and FBXL5) and VAMP8 (mediates autophagosome-lysosome fusion). NFE2L2/NRF2 knockout cells have low HERC2 expression, leading to a simultaneous increase in ferritin and NCOA4 and recruitment of apoferritin into the autophagosome. NFE2L2/NRF2 knockout cells also have low VAMP8 expression, which leads to ferritinophagy blockage. Therefore, deletion of NFE2L2/NRF2 results in apoferritin accumulation in the autophagosome, an elevated LIP, and enhanced sensitivity to ferroptosis. Concordantly, NRF2 levels correlate with HERC2 and VAMP8 in human ovarian cancer tissues, as well as ferroptosis resistance in a panel of ovarian cancer cell lines. Last, the feasibility of inhibiting NRF2 to increase the LIP and kill cancer cells via ferroptosis was demonstrated in preclinical models, signifying the impact of NRF2 inhibition in cancer treatment.
Figures


Comment in
-
Iron regulation in ferroptosis.Nat Cell Biol. 2023 Apr;25(4):515. doi: 10.1038/s41556-023-01129-5. Nat Cell Biol. 2023. PMID: 37059879 No abstract available.
References
-
- D. D. Zhang, Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 38, 769–789 (2006). - PubMed
-
- Y. Mitsuishi, K. Taguchi, Y. Kawatani, T. Shibata, T. Nukiwa, H. Aburatani, M. Yamamoto, H. Motohashi, Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22, 66–79 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

