The ion transporter Na+-K+-ATPase enables pathological B cell survival in the kidney microenvironment of lupus nephritis
- PMID: 36724234
- PMCID: PMC9891690
- DOI: 10.1126/sciadv.adf8156
The ion transporter Na+-K+-ATPase enables pathological B cell survival in the kidney microenvironment of lupus nephritis
Abstract
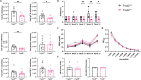
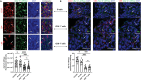
The kidney is a comparatively hostile microenvironment characterized by highsodium concentrations; however, lymphocytes infiltrate and survive therein in autoimmune diseases such as lupus. The effects of sodium-lymphocyte interactions on tissue injury in autoimmune diseases and the mechanisms used by infiltrating lymphocytes to survive the highsodium environment of the kidney are not known. Here, we show that kidney-infiltrating B cells in lupus adapt to elevated sodium concentrations and that expression of sodium potassium adenosine triphosphatase (Na+-K+-ATPase) correlates with the ability of infiltrating cells to survive. Pharmacological inhibition of Na+-K+-ATPase and genetic knockout of Na+-K+-ATPase γ subunit resulted in reduced B cell infiltration into kidneys and amelioration of proteinuria. B cells in human lupus nephritis biopsies also had high expression of Na+-K+-ATPase. Our study reveals that kidney-infiltrating B cells in lupus initiate a tissue adaption program in response to sodium stress and identifies Na+-K+-ATPase as an organ-specific therapeutic target.
Figures





Comment in
-
Mechanism of B cell survival in lupus nephritis.Nat Rev Nephrol. 2023 Apr;19(4):213. doi: 10.1038/s41581-023-00696-y. Nat Rev Nephrol. 2023. PMID: 36849741 No abstract available.
-
Mechanism of B cell survival in lupus nephritis.Nat Rev Rheumatol. 2023 Apr;19(4):196. doi: 10.1038/s41584-023-00941-5. Nat Rev Rheumatol. 2023. PMID: 36899080 No abstract available.
References
-
- L. Couzi, P. Merville, C. Deminière, J.-F. Moreau, C. Combe, J.-L. Pellegrin, J.-F. Viallard, P. Blanco, Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum. 56, 2362–2370 (2007). - PubMed
-
- A. Arazi, D. A. Rao, C. C. Berthier, A. Davidson, Y. Liu, P. J. Hoover, A. Chicoine, T. M. Eisenhaure, A. H. Jonsson, S. Li, D. J. Lieb, F. Zhang, K. Slowikowski, E. P. Browne, A. Noma, D. Sutherby, S. Steelman, D. E. Smilek, P. Tosta, W. Apruzzese, E. Massarotti, M. Dall’Era, M. Park, D. L. Kamen, R. A. Furie, F. Payan-Schober, W. F. Pendergraft III, E. A. McInnis, J. P. Buyon, M. A. Petri, C. Putterman, K. C. Kalunian, E. S. Woodle, J. A. Lederer, D. A. Hildeman, C. Nusbaum, S. Raychaudhuri, M. Kretzler, J. H. Anolik, M. B. Brenner, D. Wofsy, N. Hacohen, B. Diamond; Accelerating Medicines Partnership in SLE network , The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

