Losartan controls immune checkpoint blocker-induced edema and improves survival in glioblastoma mouse models
- PMID: 36724255
- PMCID: PMC9963691
- DOI: 10.1073/pnas.2219199120
Losartan controls immune checkpoint blocker-induced edema and improves survival in glioblastoma mouse models
Abstract
Immune checkpoint blockers (ICBs) have failed in all phase III glioblastoma trials. Here, we found that ICBs induce cerebral edema in some patients and mice with glioblastoma. Through single-cell RNA sequencing, intravital imaging, and CD8+ T cell blocking studies in mice, we demonstrated that this edema results from an inflammatory response following antiprogrammed death 1 (PD1) antibody treatment that disrupts the blood-tumor barrier. Used in lieu of immunosuppressive corticosteroids, the angiotensin receptor blocker losartan prevented this ICB-induced edema and reprogrammed the tumor microenvironment, curing 20% of mice which increased to 40% in combination with standard of care treatment. Using a bihemispheric tumor model, we identified a "hot" tumor immune signature prior to losartan+anti-PD1 therapy that predicted long-term survival. Our findings provide the rationale and associated biomarkers to test losartan with ICBs in glioblastoma patients.
Keywords: biomarkers; glioblastoma; immune checkpoint blockers; immune-related adverse events; tumor microenvironment.
Conflict of interest statement
The authors have organizational affiliations, stock ownership, and patent filings to disclose. S.C. is consultant at Guidepoint and Coleman Research. K.E.E. has intellectual property rights at NordicNeuroLab AS, Bergen, NO. M.C.S. is a current employee of GlaxoSmithKline and may own GSK stock. M.R.N. is a current employee of AbbVie and may own AbbVie stock. D.A.R. received research support from the following (paid to Dana-Farber Cancer Institute): Acerta Phamaceuticals, Agenus, Bristol-Myers Squibb, Celldex, EMD Serono, Enterome, Epitopoietic Research Coorporatioin, Incyte, Inovio, Insightec, Novartis, Omniox, and Tragara. D.A.R. received advisory/consultant fees from Abbvie; Advantagene; Agenus; Agios; Amgen; AnHeart Therapeutics; Bayer; Boston Biomedical; Boehringer Ingelheim; Bristol-Myers Squibb; Celldex; Deciphera; Del Mar Pharma; DNAtrix; Ellipses Pharma; EMD Serono; Genenta; Genentech/Roche; Hoffman-LaRoche, Ltd.; Imvax; Inovio; Kintara; Kiyatec; Medicenna Biopharma, Inc.; Merck; Merck KGaA; Monteris; Neuvogen; Novartis; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Sumitono Dainippon Pharma; Pyramid; Taiho Oncology, Inc.; and Y-mabs Therapeutics. A.H.S. has patents/pending royalties on the PD-1 pathway from Roche and Novartis. A.H.S. is on advisory boards for Surface Oncology, SQZ Biotechnologies, Elpiscience, Selecta, Bicara, Monopteros, GlaxoSmithKline, and Janssen. A.H.S. has received research funding from Novartis, Roche, UCB, Ipsen, Merck, and AbbVie unrelated to this project. G.J.F. has patents/pending royalties on the PD-1/PD-L1 pathway from Roche, Merck MSD, Bristol-Myers-Squibb, Merck KGA, Boehringer-Ingelheim, AstraZeneca, Dako, Leica, Mayo Clinic, and Novartis. G.J.F. has served on advisory boards for Roche, Bristol-Myers-Squibb, Xios, Origimed, Triursus, iTeos, NextPoint, IgM, Jubilant, Trillium, GV20, IOME, and Geode. G.J.F. has equity in Nextpoint, Triursus, Xios, iTeos, IgM, Trillium, Invaria, GV20, and Geode. M.L.S. is an equity holder, scientific cofounder, and advisory board member of Immunitas Therapeutics. R.K.J. received consultant fees from Bristol Myers Squibb (BMS), Cur Therapeutics, Elpis, Innocoll, SPARC, and SynDevRx; owns equity in Accurius, Enlight, and SynDevRx; Board of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund; and received research grants from Boehringer Ingelheim and Sanofi. No funding or reagents from these organizations were used in this study. M.Datta., L.X., M.L.S., and R.K.J. are coinventors of a patent application filed at the US Patent Office by Massachusetts General Hospital on, "Preventing immunotherapy-induced edema using angiotensin receptor blockers."
Figures

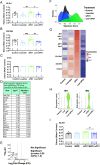



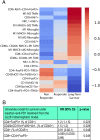
References
-
- Carpentier A. F., et al. , Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur. J. Neurol. 19, 1337–1342 (2012). - PubMed
-
- Kourilsky A., et al. , Impact of angiotensin-II receptor blockers on vasogenic edema in glioblastoma patients. J. Neurol. 263, 524–530 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K22 CA258410/CA/NCI NIH HHS/United States
- R01 NS118929/NS/NINDS NIH HHS/United States
- R01 CA259253/CA/NCI NIH HHS/United States
- R01 CA258763/CA/NCI NIH HHS/United States
- R35 CA197743/CA/NCI NIH HHS/United States
- R35 GM151041/GM/NIGMS NIH HHS/United States
- R01 CA208205/CA/NCI NIH HHS/United States
- P01 CA236749/CA/NCI NIH HHS/United States
- R37 CA245523/CA/NCI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- U01 CA224348/CA/NCI NIH HHS/United States
- R01 CA269672/CA/NCI NIH HHS/United States
- U01 CA261842/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

