Tumor-associated nonmyelinating Schwann cell-expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion
- PMID: 36724291
- PMCID: PMC9891701
- DOI: 10.1126/sciadv.add6995
Tumor-associated nonmyelinating Schwann cell-expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion
Abstract
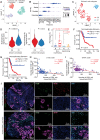
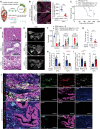
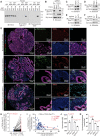
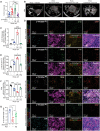
One of the major obstacles to treating pancreatic ductal adenocarcinoma (PDAC) is its immunoresistant microenvironment. The functional importance and molecular mechanisms of Schwann cells in PDAC remains largely elusive. We characterized the gene signature of tumor-associated nonmyelinating Schwann cells (TASc) in PDAC and indicated that the abundance of TASc was correlated with immune suppressive tumor microenvironment and the unfavorable outcome of patients with PDAC. Depletion of pancreatic-specific TASc promoted the tumorigenesis of PDAC tumors. TASc-expressed long noncoding RNA (lncRNA) plasmacytoma variant translocation 1 (PVT1) was triggered by the tumor cell-produced interleukin-6. Mechanistically, PVT1 modulated RAF proto-oncogene serine/threonine protein kinase-mediated phosphorylation of tryptophan 2,3-dioxygenase in TASc, facilitating its enzymatic activities in catalysis of tryptophan to kynurenine. Depletion of TASc-expressed PVT1 suppressed PDAC tumor growth. Furthermore, depletion of TASc using a small-molecule inhibitor effectively sensitized PDAC to immunotherapy, signifying the important roles of TASc in PDAC immune resistance.
Figures


References
-
- Dey P., Li J., Zhang J., Chaurasiya S., Strom A., Wang H., Liao W. T., Cavallaro F., Denz P., Bernard V., Yen E. Y., Genovese G., Gulhati P., Liu J., Chakravarti D., Deng P., Zhang T., Carbone F., Chang Q., Ying H., Shang X., Spring D. J., Ghosh B., Putluri N., Maitra A., Wang Y. A., DePinho R. A., Oncogenic KRAS-driven metabolic reprogramming in pancreatic cancer cells utilizes cytokines from the tumor microenvironment. Cancer Discov. 10, 608–625 (2020). - PMC - PubMed
-
- Demir I. E., Friess H., Ceyhan G. O., Neural plasticity in pancreatitis and pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 12, 649–659 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

