Combined Statin and Glucocorticoid Therapy for the Safer Treatment of Preterm Birth
- PMID: 36724801
- PMCID: PMC10017302
- DOI: 10.1161/HYPERTENSIONAHA.122.19647
Combined Statin and Glucocorticoid Therapy for the Safer Treatment of Preterm Birth
Abstract
Background: Prematurity is strongly associated with poor respiratory function in the neonate. Rescue therapies include treatment with glucocorticoids due to their anti-inflammatory and maturational effects on the developing lung. However, glucocorticoid treatment in the infant can increase the risk of long-term cardiovascular complications including hypertension, cardiac, and endothelial dysfunction. Accumulating evidence implicates a molecular link between glucocorticoid excess and depletion of nitric oxide (NO) bioavailability as a mechanism underlying the detrimental effects of postnatal steroids on the heart and circulation. Therefore, combined glucocorticoid and statin therapy, by increasing NO bioavailability, may protect the developing cardiovascular system while maintaining beneficial effects on the lung.
Methods: We investigated combined glucocorticoid and statin therapy using an established rodent model of prematurity and combined experiments of cardiovascular function in vivo, with those in isolated organs as well as measurements at the cellular and molecular levels.
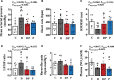
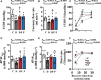
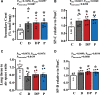
Results: We show that neonatal glucocorticoid treatment increases the risk of later cardiovascular dysfunction in the offspring. Underlying mechanisms include decreased circulating NO bioavailability, sympathetic hyper-reactivity, and NO-dependent endothelial dysfunction. Combined neonatal glucocorticoid and statin therapy protects the developing cardiovascular system by normalizing NO and sympathetic signaling, without affecting pulmonary maturational or anti-inflammatory effects of glucocorticoids.
Conclusions: Therefore, combined glucocorticoid and statin therapy may be safer than glucocorticoids alone for the treatment of preterm birth.
Keywords: cardiovascular diseases; glucocorticoids; hypertension; infant; newborn; premature birth.
Figures


References
-
- Halliday H, Ehrenkranz R, Doyle L. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2010;1:CD001146. doi: 10.1002/14651858.CD001146.pub2 - PubMed
-
- Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/nejm196702162760701 - PubMed
-
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. doi: 10.1542/peds.50.4.515 - PubMed
-
- Liggins G. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45:515–523. doi: 10.1677/joe.0.0450515 - PubMed
-
- Cummings JJ, D’Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med. 1989;320:1505–1510. doi: 10.1056/nejm198906083202301 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

