Mechanosensitive ion channels in apoptosis and ferroptosis: focusing on the role of Piezo1
- PMID: 36724905
- PMCID: PMC10068349
- DOI: 10.5483/BMBRep.2023-0002
Mechanosensitive ion channels in apoptosis and ferroptosis: focusing on the role of Piezo1
Abstract
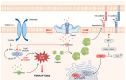
Mechanosensitive ion channels sense mechanical stimuli applied directly to the cellular membranes or indirectly through their tethered components, provoking cellular mechanoresponses. Among others, Piezo1 mechanosensitive ion channel is a relatively novel Ca2+-permeable channel that is primarily present in non-sensory tissues. Recent studies have demonstrated that Piezo1 plays an important role in Ca2+-dependent cell death, including apoptosis and ferroptosis, in the presence of mechanical stimuli. It has also been proven that cancer cells are sensitive to mechanical stresses due to higher expression levels of Piezo1 compared to normal cells. In this review, we discuss Piezo1-mediated cell death mechanisms and therapeutic strategies to inhibit or induce cell death by modulating the activity of Piezo1 with pharmacological drugs or mechanical perturbations induced by stretch and ultrasound. [BMB Reports 2023; 56(3): 145-152].
Conflict of interest statement
The authors have no conflicting interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

