Clinical Significance of Liver MR Imaging
- PMID: 36725068
- PMCID: PMC10086396
- DOI: 10.2463/mrms.rev.2022-0100
Clinical Significance of Liver MR Imaging
Abstract
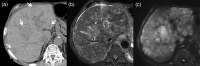
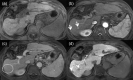
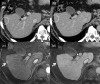
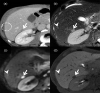
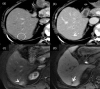
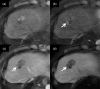
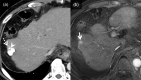
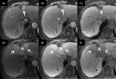
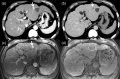
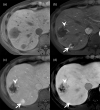
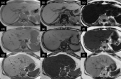
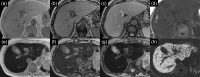
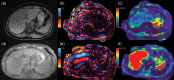
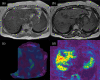
MRI is widely used in clinical practice for detecting liver diseases. Since the introduction of gadoxetic acid, MRI has become the most effective modality for the detection and characterization of focal liver lesions. According to previous meta-analyses, the area under the receiver operating characteristic curve (AUROC) was 0.97-0.99 for the diagnosis of small hepatocellular carcinoma (≥ 2 cm) by gadoxetic-acid-enhanced MRI. Moreover, the AUROC for the diagnosis of colorectal liver metastases was significantly high (0.98). Despite gadoxetic acid's drawbacks, its clinical utility outweighs them, making it the contrast agent of choice in routine liver MRIs. Moreover, clinically, liver MRI has become more prevalent for a quantitative assessment. Liver fibrosis can be evaluated using MR elastography; whereas, hepatic steatosis and iron overload can be evaluated using proton density fat fraction, with high accuracy and reproducibility. This article reviewed the usefulness of liver MRI, which can be a comprehensive imaging modality in clinical practice.
Keywords: elastography; gadoxetic acid; liver; magnetic resonance imaging; proton density fat fraction.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Donato H, França M, Candelária I, Caseiro-Alves F. Liver MRI: from basic protocol to advanced techniques. Eur J Radiol 2017; 93:30–39. - PubMed
-
- Ichikawa S, Morisaka H, Omiya Y, Onishi H. Distinction between hepatocellular carcinoma and hypervascular liver metastases in non-cirrhotic patients using gadoxetate disodium-enhanced magnetic resonance imaging. Can Assoc Radiol J 2022; 73:639–646. - PubMed

