Systematic, comprehensive, evidence-based approach to identify neuroprotective interventions for motor neuron disease: using systematic reviews to inform expert consensus
- PMID: 36725099
- PMCID: PMC9896226
- DOI: 10.1136/bmjopen-2022-064169
Systematic, comprehensive, evidence-based approach to identify neuroprotective interventions for motor neuron disease: using systematic reviews to inform expert consensus
Abstract
Objectives: Motor neuron disease (MND) is an incurable progressive neurodegenerative disease with limited treatment options. There is a pressing need for innovation in identifying therapies to take to clinical trial. Here, we detail a systematic and structured evidence-based approach to inform consensus decision making to select the first two drugs for evaluation in Motor Neuron Disease-Systematic Multi-arm Adaptive Randomised Trial (MND-SMART: NCT04302870), an adaptive platform trial. We aim to identify and prioritise candidate drugs which have the best available evidence for efficacy, acceptable safety profiles and are feasible for evaluation within the trial protocol.
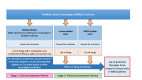
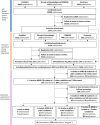
Methods: We conducted a two-stage systematic review to identify potential neuroprotective interventions. First, we reviewed clinical studies in MND, Alzheimer's disease, Huntington's disease, Parkinson's disease and multiple sclerosis, identifying drugs described in at least one MND publication or publications in two or more other diseases. We scored and ranked drugs using a metric evaluating safety, efficacy, study size and study quality. In stage two, we reviewed efficacy of drugs in MND animal models, multicellular eukaryotic models and human induced pluripotent stem cell (iPSC) studies. An expert panel reviewed candidate drugs over two shortlisting rounds and a final selection round, considering the systematic review findings, late breaking evidence, mechanistic plausibility, safety, tolerability and feasibility of evaluation in MND-SMART.
Results: From the clinical review, we identified 595 interventions. 66 drugs met our drug/disease logic. Of these, 22 drugs with supportive clinical and preclinical evidence were shortlisted at round 1. Seven drugs proceeded to round 2. The panel reached a consensus to evaluate memantine and trazodone as the first two arms of MND-SMART.
Discussion: For future drug selection, we will incorporate automation tools, text-mining and machine learning techniques to the systematic reviews and consider data generated from other domains, including high-throughput phenotypic screening of human iPSCs.
Keywords: Adult neurology; Clinical trials; Motor neurone disease; NEUROLOGY; THERAPEUTICS.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: In the last 3 years, JC has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust. He is supported in part by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in MS funded by the Canadian MS society. A local principal investigator for commercial trials funded by: Actelion, Novartis and Roche; and has taken part in advisory boards/consultancy for Azadyne, Janssen, Merck, NervGen, Novartis and Roche.
Figures
References
-
- European Medicines Agency . In: Agency EM, ed. Refusal of the marketing authorisation for Alsitek (masitinib). 2018.
-
- European Medicines Agency . Withdrawal assessment report radicava (international non-proprietary name: edaravone). In: Procedure no.EMEA/H/C/004938/0000. Amsterdam, The Netherlands, 2019.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
