Lung specific homing of diphenyleneiodonium chloride improves pulmonary fibrosis by inhibiting macrophage M2 metabolic program
- PMID: 36725191
- PMCID: PMC9936526
- DOI: 10.1016/j.jare.2022.04.012
Lung specific homing of diphenyleneiodonium chloride improves pulmonary fibrosis by inhibiting macrophage M2 metabolic program
Erratum in
-
Corrigendum to "Lung-specific homing of diphenyleneiodonium chloride improves pulmonary fibrosis by inhibiting macrophage M2 metabolic program" [J. Adv. Res. 44 (2023) 213-225].J Adv Res. 2024 May;59:223. doi: 10.1016/j.jare.2024.04.006. J Adv Res. 2024. PMID: 38692745 Free PMC article. No abstract available.
Abstract
Introduction: Pulmonary fibrosis (PF) is a fatal disease with a variable and unpredictable course. Effective clinical treatment for PF remains a challenge due to low drug accumulation in lungs and imbalanced polarization of pro/anti-fibrotic macrophages.
Objectives: To identify the alteration of immunometabolism in the pulmonary macrophages and investigate the feasibility of specific inhibition of M2 activation of macrophages as an effective anti-PF strategy in vivo.
Methods: The high-content screening system was used to select lung-specific homing compounds that can modulate macrophage polarization. Imaging mass spectrometry (IMS) conjugated with chemical proteomics approach was conducted to explore the cells and proteins targeted by diphenyleneiodonium chloride (DPI). A bleomycin-induced fibrotic mouse model was established to examine the in vivo effect of DPI.
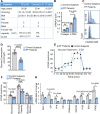
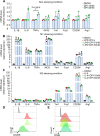
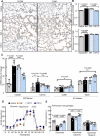
Results: Pulmonary macrophages of PF at late stage exhibited predominantly the M2 phenotype with decreased glycolysis metabolism. DPI was demonstrated to inhibit profibrotic activation of macrophages in the preliminary screening. Notably, IMS conjugated with chemical proteomics approach revealed DPI specifically targeted pulmonary macrophages, leading to the efficient protection from bleomycin-induced pulmonary fibrosis in mice. Mechanistically, DPI upregulated glycolysis and suppressed M2 programming in fibrosis mice, thus resulting in pro-fibrotic cytokine inhibition, hydroxyproline biosynthesis, and collagen deposition, with a concomitant increase in alveolar airspaces.
Conclusions: DPI mediated glycolysis in lung and accordingly suppressed M2 programming, resulting in improved lung fibrosis.
Keywords: Chemical proteomics; Diphenyleneiodonium chloride; Imaging mass spectrometry; Lung fibrosis.
Copyright © 2022. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Ferkol T., Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11(3):404–406. - PubMed
-
- Wilczewska A.Z., Niemirowicz K., Markiewicz K.H., Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–1037. - PubMed
-
- Newman S.P. Drug delivery to the lungs: challenges and opportunities. Ther Deliv. 2017;8(8):647–661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

