Engineered Sensory Nerve Guides Self-Adaptive Bone Healing via NGF-TrkA Signaling Pathway
- PMID: 36725311
- PMCID: PMC10074090
- DOI: 10.1002/advs.202206155
Engineered Sensory Nerve Guides Self-Adaptive Bone Healing via NGF-TrkA Signaling Pathway
Abstract
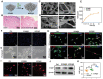
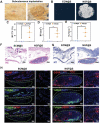
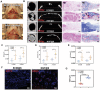
The upstream role of sensory innervation during bone homeostasis is widely underestimated in bone repairing strategies. Herein, a neuromodulation approach is proposed to orchestrate bone defect healing by constructing engineered sensory nerves (eSN) in situ to leverage the adaptation feature of SN during tissue formation. NGF liberated from ECM-constructed eSN effectively promotes sensory neuron differentiation and enhances CGRP secretion, which lead to improved RAOECs mobility and osteogenic differentiation of BMSC. In turn, such eSN effectively drives ossification in vivo via NGF-TrkA signaling pathway, which substantially accelerates critical size bone defect healing. More importantly, eSN also adaptively suppresses excessive bone formation and promotes bone remodeling by activating osteoclasts via CGRP-dependent mechanism when combined with BMP-2 delivery, which ingeniously alleviates side effects of BMP-2. In sum, this eSN approach offers a valuable avenue to harness the adaptive role of neural system to optimize bone homeostasis under various clinical scenario.
Keywords: BMP-2; extracellular matrix; nerve growth factor; osteogenesis; sensory nerve.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Rupp M., Biehl C., Budak M., Thormann U., Heiss C., Alt V., Int. Orthop. 2018, 42, 247; - PubMed
- b) Stewart S. K., Malays Orthop. J. 2019, 13, 1.
-
- a) Cai B., Lin D., Li Y., Wang L., Xie J., Dai T., Liu F., Tang M., Tian L., Yuan Y., Kong L., Shen S. G. F., Adv. Sci. 2021, 8, 2100584; - PMC - PubMed
- b) Mahon O. R., Browe D. C., Gonzalez‐Fernandez T., Pitacco P., Whelan I. T., Euw S. V., Hobbs C., Nicolosi V., Cunningham K. T., Mills K. H. G., Kelly D. J., Dunne A., Biomaterials 2020, 239, 119833; - PubMed
- c) Jin S.‐S., He D.‐Q., Luo D., Wang Y., Yu M., Guan B., Fu Y., Li Z.‐X., Zhang T., Zhou Y.‐H., Wang C.‐Y., Liu Y., ACS Nano 2019, 13, 6581; - PubMed
- d) Zhao D. W., Zuo K. Q., Wang K., Sun Z. Y., Lu Y. P., Cheng L., Xiao G. Y., Liu C., Mater. Sci. Eng., C 2021, 118, 111512. - PMC - PubMed
-
- a) Abeynayake N., Arthur A., Gronthos S., Bone 2021, 142, 115645; - PubMed
- b) Hu C.‐H., Sui B.‐D., Liu J., Dang L., Chen J., Zheng C.‐X., Shi S., Zhao N., Dang M.‐Y., He X.‐N., Zhang L.‐Q., Gao P.‐P., Chen N., Kuang H.‐J., Chen K., Xu X.‐L., Yu X.‐R., Zhang G., Jin Y., Small Methods 2022, 6, 2100763; - PubMed
- c) Chen H., Hu B., Lv X., Zhu S., Zhen G., Wan M., Jain A., Gao B., Chai Y., Yang M., Wang X., Deng R., Wang L., Cao Y., Ni S., Liu S., Yuan W., Chen H., Dong X., Guan Y., Yang H., Cao X., Nat. Commun. 2019, 10, 181; - PMC - PubMed
- d) Kondo H., Kondo M., Hayashi K., Kusafuka S., Hamamura K., Tanaka K., Kodama D., Hirai T., Sato T., Ariji Y., Miyazawa K., Ariji E., Goto S., Togari A., Eur. J. Orthod. 2022, 44, 404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
