Vedolizumab is superior to infliximab in biologic naïve patients with ulcerative colitis
- PMID: 36725872
- PMCID: PMC9892496
- DOI: 10.1038/s41598-023-28907-3
Vedolizumab is superior to infliximab in biologic naïve patients with ulcerative colitis
Abstract
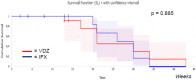
There are no prospective, head-to-head, controlled trials comparing the efficacy and safety of Infliximab (IFX) and Vedolizumab (VDZ) for the treatment of moderate-to-severe ulcerative colitis (UC), while only a few real-life retrospective studies have been published so far. We assessed the efficacy of IFX vs. VDZ in two cohorts of biologic-naïve outpatients with moderate-to-severe UC or mild, but refractory, disease. Data were extracted from patients' files and reviewed. The duration of follow-up (FU) was 52 weeks. The primary endpoint was the clinical remission (CR) at the end of FU. Secondary endpoints were: drug persistency, time to obtain CR, clinical response at the end of the induction phase (IP), steroid-free CR (compared to patients who used steroids at baseline) at the end of FU, need for drug optimization, adverse events (AEs), and normalization of C-reactive protein (CRP). We also analyzed the causes of dropping out (primary non-response), or secondary loss of response (immunogenic or not), for each group. We enrolled 82 patients (50 IFX and 32 VDZ) who met the inclusion criteria. At the end of FU, CR was obtained in 32% of the patients on IFX and 75% on VDZ (p = 0.0003). Drug persistency was superior for VDZ compared to IFX (78% vs. 52%, p = 0.033). Clinical response at the end of induction was reached in 54% and in 81% in the IFX and VDZ group, respectively (p = 0.014). Steroid-free clinical remission at the end of FU was 62% and 94% in the IFX vs. VDZ group, respectively (p = 0.036). The need for drug optimization was higher for VDZ than for IFX (28% vs. 57%, p = 0.009), while the time to obtain CR, the incidence of AEs, mean duration of FU, and rate of CRP normalization at the end of IP were comparable between the two groups. There was a prevalence of patients dropping out because of primary non-response in IFX group (p = 0.027), while the incidence of secondary loss of response was similar in the two groups. At the multivariate analysis, CRP and Partial Mayo Score (PMS) at T0 did not correlate with CR at the end of FU in both groups. In this retrospective, real world data study in biologic-naïve patients, VDZ was superior to IFX in CR, clinical response rate at the end of IP, drug persistency, steroid-free remission, and need for optimization at the end of FU.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A. Quality of life in inflammatory bowel disease: A systematic review and metaanalyses-part I. Inflamm. Bowel Dis. 2018;24(4):742–751. - PubMed
-
- Valpiani D, Manzi I, Mercuriali M, Giuliani O, Ravaioli A, Colamartini A, Bucchi L, Falcini F, Ricci E. A model of inflammatory bowel disease population-based registry: The Forlì experience (1993–2013) Dig. Liver Dis. 2018;50(1):32–36. - PubMed
-
- Macaluso FS, Mocci G, Orlando A, Scondotto S, Fantaci G, Antonello A, Leone S, Previtali E, Cabras F, Cottone M. Prevalence and incidence of inflammatory bowel disease in two Italian islands, Sicily and Sardinia: A report based on health information systems. Dig. Liver Dis. 2019;51(9):1270–1274. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous