Structural basis for substrate selection by the SARS-CoV-2 replicase
- PMID: 36725929
- PMCID: PMC9891196
- DOI: 10.1038/s41586-022-05664-3
Structural basis for substrate selection by the SARS-CoV-2 replicase
Abstract
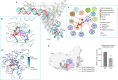
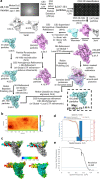
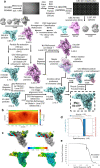
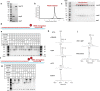
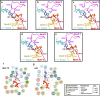
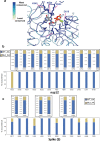
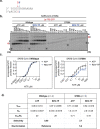
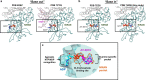
The SARS-CoV-2 RNA-dependent RNA polymerase coordinates viral RNA synthesis as part of an assembly known as the replication-transcription complex (RTC)1. Accordingly, the RTC is a target for clinically approved antiviral nucleoside analogues, including remdesivir2. Faithful synthesis of viral RNAs by the RTC requires recognition of the correct nucleotide triphosphate (NTP) for incorporation into the nascent RNA. To be effective inhibitors, antiviral nucleoside analogues must compete with the natural NTPs for incorporation. How the SARS-CoV-2 RTC discriminates between the natural NTPs, and how antiviral nucleoside analogues compete, has not been discerned in detail. Here, we use cryogenic-electron microscopy to visualize the RTC bound to each of the natural NTPs in states poised for incorporation. Furthermore, we investigate the RTC with the active metabolite of remdesivir, remdesivir triphosphate (RDV-TP), highlighting the structural basis for the selective incorporation of RDV-TP over its natural counterpart adenosine triphosphate3,4. Our results explain the suite of interactions required for NTP recognition, informing the rational design of antivirals. Our analysis also yields insights into nucleotide recognition by the nsp12 NiRAN (nidovirus RdRp-associated nucleotidyltransferase), an enigmatic catalytic domain essential for viral propagation5. The NiRAN selectively binds guanosine triphosphate, strengthening proposals for the role of this domain in the formation of the 5' RNA cap6.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
E.A.C. and S.A.D. received funding from Gilead Sciences, Inc. in support of this study. J.K.P., T.C.A., J.Y.F. and J.P.B. are Gilead employees. M.G. received funding from Gilead Sciences Inc. for studies on the mechanism of action of remdesivir. All other authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

