Neuronal surface antigen-specific immunostaining pattern on a rat brain immunohistochemistry in autoimmune encephalitis
- PMID: 36726989
- PMCID: PMC9885155
- DOI: 10.3389/fimmu.2022.1066830
Neuronal surface antigen-specific immunostaining pattern on a rat brain immunohistochemistry in autoimmune encephalitis
Abstract
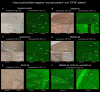
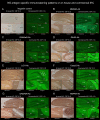
A variety of neuronal surface (NS) antibodies (NS-Ab) have been identified in autoimmune encephalitis (AE). Tissue-based assay (TBA) using a rodent brain immunohistochemistry (IHC) is used to screen NS-Ab, while cell-based assay (CBA) to determine NS antigens. Commercial rat brain IHC is currently available but its clinical relevance remains unclear. Immunostaining patterns of NS antigens have not been extensively studied yet. To address these issues, we assessed a predictive value of "neuropil pattern" and "GFAP pattern" on commercial IHC in 261 patients, and characterized an immunostaining pattern of 7 NS antigens (NMDAR, LGI1, GABAaR, GABAbR, AMPAR, Caspr2, GluK2). Sensitivity and specificity of "neuropil pattern" for predicting NS-Ab were 66.0% (95% CI 55.7-75.3), and 98.2% (95% CI 94.8-99.6), respectively. False-positive rate was 1.8% (3/164) while false-negative rate was 34.0% (33/97). In all 3 false-positive patients, neuropil-like staining was attributed to high titers of GAD65-Ab. In 33 false-negative patients, NMDAR was most frequently identified (n=18 [54.5%], 16/18 [88.9%] had low titers [< 1:32]), followed by GABAaR (n=5). Of 261 patients, 25 (9.6%) had either GFAP (n=21) or GFAP-mimicking pattern (n=4). GFAP-Ab were identified in 21 of 31 patients examined with CBA (20 with GFAP pattern, 1 with GFAP-mimicking pattern). Immunostaining pattern of each NS antigen was as follows: 1) NMDAR revealed homogenous reactivity in the dentate gyrus molecular layer (DG-ML) with less intense dot-like reactivity in the cerebellar granular layer (CB-GL); 2) both GABAaR and GluK2 revealed intense dot-like reactivity in the CB-GL, but GABAaR revealed homogenous reactivity in the DG-ML while GluK2 revealed intense reactivity along the inner layer of the DG-ML; and 3) LGI1, Caspr2, GABAbR, and AMPAR revealed intense reactivity in the cerebellar ML (CB-ML) but LGI1 revealed intense reactivity along the middle layer of the DG-ML. Whereas, Caspr2, GABAbR, and AMPAR revealed similar reactivity in the DG-ML but some difference in other regions. TBA is useful not only for screening NS- or GFAP-Ab but also for estimating NS antigens; however, negative results should be interpreted cautiously because "neuropil pattern" may be missed on commercial IHC when antibody titers are low. Antigen-specific immunoreactivity is a useful biomarker of AE.
Keywords: autoantibodies; autoimmune encephalitis; cell-based assay; immunohistochemistry; neuronal surface antigens; tissue-based assay.
Copyright © 2023 Nagata, Kanazawa, Mitsuhata, Iizuka, Nagashima, Nakamura, Kaneko, Kitamura, Nishiyama and Iizuka.
Conflict of interest statement
KN received research supports from Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., and Eisai Co., Ltd. TI received a research support from Astellas Pharma Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Dalmau J, Graus F. Autoimmune encephalitis and related disorders of the nervous system. In: Autoimmune encephalitis and related disorders of the nervous system (pp. I-ii). (Cambridge: Cambridge University Press; ) (2022).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

