Pediatric diffuse midline glioma H3K27- altered: A complex clinical and biological landscape behind a neatly defined tumor type
- PMID: 36727064
- PMCID: PMC9885151
- DOI: 10.3389/fonc.2022.1082062
Pediatric diffuse midline glioma H3K27- altered: A complex clinical and biological landscape behind a neatly defined tumor type
Abstract
The 2021 World Health Organization Classification of Tumors of the Central Nervous System, Fifth Edition (WHO-CNS5), has strengthened the concept of tumor grade as a combination of histologic features and molecular alterations. The WHO-CNS5 tumor type "Diffuse midline glioma, H3K27-altered," classified within the family of "Pediatric-type diffuse high-grade gliomas," incarnates an ideally perfect integrated diagnosis in which location, histology, and genetics clearly define a specific tumor entity. It tries to evenly characterize a group of neoplasms that occur primarily in children and midline structures and that have a dismal prognosis. Such a well-defined pathological categorization has strongly influenced the pediatric oncology community, leading to the uniform treatment of most cases of H3K27-altered diffuse midline gliomas (DMG), based on the simplification that the mutation overrides the histological, radiological, and clinical characteristics of such tumors. Indeed, multiple studies have described pediatric H3K27-altered DMG as incurable tumors. However, in biology and clinical practice, exceptions are frequent and complexity is the rule. First of all, H3K27 mutations have also been found in non-diffuse gliomas. On the other hand, a minority of DMGs are H3K27 wild-type but have a similarly poor prognosis. Furthermore, adult-type tumors may rarely occur in children, and differences in prognosis have emerged between adult and pediatric H3K27-altered DMGs. As well, tumor location can determine differences in the outcome: patients with thalamic and spinal DMG have significantly better survival. Finally, other concomitant molecular alterations in H3K27 gliomas have been shown to influence prognosis. So, when such additional mutations are found, which one should we focus on in order to make the correct clinical decision? Our review of the current literature on pediatric diffuse midline H3K27-altered DMG tries to address such questions. Indeed, H3K27 status has become a fundamental supplement to the histological grading of pediatric gliomas; however, it might not be sufficient alone to exhaustively define the complex biological behavior of DMG in children and might not represent an indication for a unique treatment strategy across all patients, irrespective of age, additional molecular alterations, and tumor location.
Keywords: CNS tumors; H3K27; WHO CNS 5; WHO classification; brain cancer; diffuse midline glioma; pediatric; pediatric neuro-oncology.
Copyright © 2023 Vallero, Bertero, Morana, Sciortino, Bertin, Mussano, Ricci, Peretta and Fagioli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

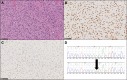
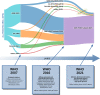
References
-
- Pfister SM, Reyes-Múgica M, Chan JKC, Hasle H, Lazar AJ, Rossi S, et al. A summary of the inaugural WHO classification of pediatric tumors: Transitioning from the optical into the molecular era. Cancer Discovery (2022) 12:331–55. doi: 10.1158/2159-8290.CD-21-1094 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

