Somatic genetic alterations predict hematological progression in GATA2 deficiency
- PMID: 36727400
- PMCID: PMC10230419
- DOI: 10.3324/haematol.2022.282250
Somatic genetic alterations predict hematological progression in GATA2 deficiency
Abstract
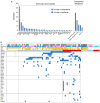
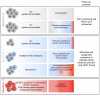
Germline GATA2 mutations predispose to myeloid malignancies resulting from the progressive acquisition of additional somatic mutations. Here we describe clinical and biological features of 78 GATA2-deficient patients. Hematopoietic stem and progenitor cell phenotypic characterization revealed an exhaustion of myeloid progenitors. Somatic mutations in STAG2, ASXL1 and SETBP1 genes along with cytogenetic abnormalities (monosomy 7, trisomy 8, der(1;7)) occurred frequently in patients with GATA2 germline mutations. Patients were classified into three hematopoietic spectra based on bone marrow cytomorphology. No somatic additional mutations were detected in patients with normal bone marrow (spectrum 0), whereas clonal hematopoiesis mediated by STAG2 mutations was frequent in those with a hypocellular and/or myelodysplastic bone marrow without excess blasts (spectrum 1). Finally, SETBP1, RAS pathway and RUNX1 mutations were predominantly associated with leukemic transformation stage (spectrum 2), highlighting their implications in the transformation process. Specific somatic alterations, potentially providing distinct selective advantages to affected cells, are therefore associated with the clinical/hematological evolution of GATA2 syndrome. Our study not only suggests that somatic genetic profiling will help clinicians in their management of patients, but will also clarify the mechanism of leukemogenesis in the context of germline GATA2 mutations.
Figures





References
-
- Song WJ, Sullivan MG, Legare RD, et al. . Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23(2):166-175. - PubMed
-
- Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. Mutation of CEBPA in familial acute myeloid leukemia. N Engl J Med. 2004;351(23):2403-2407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

