Allograft Versus Bioactive Glass (BG-S53P4) in Pediatric Benign Bone Lesions: A Randomized Clinical Trial
- PMID: 36727973
- PMCID: PMC10752261
- DOI: 10.2106/JBJS.22.00716
Allograft Versus Bioactive Glass (BG-S53P4) in Pediatric Benign Bone Lesions: A Randomized Clinical Trial
Abstract
Background: Benign bone cysts in children have a high risk of recurrence after bone grafting. The optimal treatment and filling material for these lesions are currently unknown.
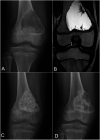
Methods: We compared cyst recurrence after intralesional curettage and filling with allograft versus bioactive glass (BG-S53P4; Bonalive) in a randomized clinical trial. The volume of recurrent cyst at 2-year follow-up was the primary outcome.
Results: Of 64 eligible children, 51 (mean age, 11.1 years) were randomized to undergo filling of the cyst using morselized allograft (26) or bioactive glass (25). Twelve (46%) of the children in the allograft group and 10 (40%) in the bioactive glass group developed a recurrence (odds ratio [OR] for bioactive glass = 0.79, 95% confidence interval [CI] = 0.25 to 2.56, p = 0.77). The size of the recurrent cyst did not differ between the allograft group (mean, 3.3 mL; range, 0 to 13.2 mL) and the bioactive glass group (mean, 2.2 mL; range, 0 to 16.6 mL, p = 0.43). After adjusting for the type of lesion (aneurysmal bone cyst versus other), bioactive glass also did not prevent larger (>1 mL) recurrent cysts (adjusted OR = 0.42, 95% CI = 0.13 to 1.40, p = 0.16). The Musculoskeletal Tumor Society score improved significantly (p ≤ 0.013) from preoperatively to the 2-year follow-up in both groups (to 28.7 for bioactive glass and 29.1 for bone graft). Four (15%) of the children in the allograft group and 6 (24%) in the bioactive glass group required a reoperation during the follow-up (OR for bioactive glass = 1.74, 95% CI = 0.43 to 7.09, p = 0.50).
Conclusions: Filling with bioactive glass and with allograft in the treatment of benign bone lesions provided comparable results in terms of recurrence and complications.
Level of evidence: Therapeutic Level I . See Instructions for Authors for a complete description of levels of evidence.
Copyright © 2023 by The Journal of Bone and Joint Surgery, Incorporated.
Conflict of interest statement
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article ( http://links.lww.com/JBJS/H409 ).
Figures


References
-
- Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. Orthopaedics & Traumatology: Surgery & Research. 2015. Feb;101(1)(Suppl):S119-27. - PubMed
-
- Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: a review of 150 patients. Journal of Clinical Oncology. 2005. Sep 20;23(27):6756-62. - PubMed
-
- Hou HY, Wu K, Wang CT, Chang SM, Lin WH, Yang RS. Treatment of unicameral bone cyst: a comparative study of selected techniques. The Journal of Bone and Joint Surgery-American Volume. 2010. Apr;92(4):855-62. - PubMed
-
- Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clinical Orthopaedics and Related Research. 363, 1999. Jun;(363):176-9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

