Limited Health Risks in Performing Drug Reconstitution and Handling Tasks in Pharmacies-Results of an Occupational Risk Assessment Study
- PMID: 36728178
- PMCID: PMC10090273
- DOI: 10.1097/JOM.0000000000002781
Limited Health Risks in Performing Drug Reconstitution and Handling Tasks in Pharmacies-Results of an Occupational Risk Assessment Study
Abstract
Objective: Some drugs need processing before they can be administered or dispensed. We measured airborne exposure of pharmacy staff to small particles when performing these tasks.
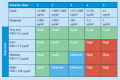
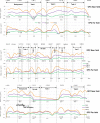
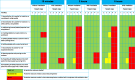
Methods: Reconstitution of powdered drugs in vials; crushing, splitting, and counting of tablets; and opening of capsules, using different ventilation strategies, were investigated in five pharmacies after in a worst-case approach. Airborne particulate matter was determined for a range of particles sizes.
Results: Mean particle concentrations ranged from not detectable to 1.03 μg/m 3 (<1 μm) and 589.7 μg/m 3 (<10 μm). Dust exhaust made tasks safer. Most hazardous was pouring out tablets from a bulk supply, and least hazardous was reconstitution of a powder for injection.
Conclusions: Occupational exposure during routine handling of drugs can occur, but the risks vary greatly with the nature and duration of the tasks.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American College of Occupational and Environmental Medicine.
Conflict of interest statement
Conflict of Interest: None declared.
Figures

References
-
- European Parliament and the Council of the European Union . Directive 92/85/EEC of the European Parliament and of the Council on the introduction of measures to encourage improvements in the safety and health at work of pregnant workers and workers who have recently given birth or are breastfeeding (tenth individual Directive within the meaning of Article 16 (1) of Directive 89/391/EEC). Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31992L0085. Accessed August 28, 2017.
-
- European Parliament and the Council of the European Union, April 7th, 1998 . Directive 98/24/EC on the protection of the health and safety of workers from the risks related to chemical agents at work (fourteenth individual Directive within the meaning of Article 16(1) of Directive 89/391/EEC). Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31998L0024. Accessed August 28, 2017.
-
- European Parliament and the Council of the European Union . Directive 2004/37/EC of the European Parliament and of the Council on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work (Sixth Individual Directive within the Meaning of Article 16[1] of Council Directive 89/391/EEC). Official Journal of the European Union April 29, 2004. 158:50–76. Available at: www.reach-compliance.eu/english/REACH-E/engine/sources/regulations/2004-.... Accessed August 28, 2017.
-
- NIOSH . Preventing Occupational Exposure to Antineoplastic and Other Hazardous Drugs in Health Care Settings. Publication Number 2004–165; 2004. Available at: https://www.cdc.gov/niosh/docs/2004-165/default.html. Accessed August 28, 2017.
-
- European Society of Oncology Pharmacy (ESOP) Quapos: Quality Standard for the Oncology Pharmacy Service. 6th ed. 2018. Available at: https://esop.li/quapos/. Accessed September 30, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

