The effects of oral ferrous bisglycinate supplementation on hemoglobin and ferritin concentrations in adults and children: a systematic review and meta-analysis of randomized controlled trials
- PMID: 36728680
- PMCID: PMC10331582
- DOI: 10.1093/nutrit/nuac106
The effects of oral ferrous bisglycinate supplementation on hemoglobin and ferritin concentrations in adults and children: a systematic review and meta-analysis of randomized controlled trials
Abstract
Context: Iron deficiency and anemia have serious consequences, especially for children and pregnant women. Iron salts are commonly provided as oral supplements to prevent and treat iron deficiency, despite poor bioavailability and frequently reported adverse side effects. Ferrous bisglycinate is a novel amino acid iron chelate that is thought to be more bioavailable and associated with fewer gastrointestinal (GI) adverse events as compared with iron salts.
Objective: A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to evaluate the effects of ferrous bisglycinate supplementation compared with other iron supplements on hemoglobin and ferritin concentrations and GI adverse events.
Data sources: A systematic search of electronic databases and grey literature was performed up to July 17, 2020, yielding 17 RCTs that reported hemoglobin or ferritin concentrations following at least 4 weeks' supplementation of ferrous bisglycinate compared with other iron supplements in any dose or frequency.
Data extraction: Random-effects meta-analyses were conducted among trials of pregnant women (n = 9) and children (n = 4); pooled estimates were expressed as standardized mean differences (SMDs). Incidence rate ratios (IRRs) were estimated for GI adverse events, using Poisson generalized linear mixed-effects models. The remaining trials in other populations (n = 4; men and nonpregnant women) were qualitatively evaluated.
Data analysis: Compared with other iron supplements, supplementation with ferrous bisglycinate for 4-20 weeks resulted in higher hemoglobin concentrations in pregnant women (SMD, 0.54 g/dL; 95% confidence interval [CI], 0.15-0.94; P < 0.01) and fewer reported GI adverse events (IRR, 0.36; 95%CI, 0.17-0.76; P < 0.01). We observed a non-significant trend for higher ferritin concentrations in pregnant women supplemented with ferrous bisglycinate. No significant differences in hemoglobin or ferritin concentrations were detected among children.
Conclusion: Ferrous bisglycinate shows some benefit over other iron supplements in increasing hemoglobin concentration and reducing GI adverse events among pregnant women. More trials are needed to assess the efficacy of ferrous bisglycinate against other iron supplements in other populations.
Prospero registration no: CRD42020196984.
Keywords: anemia; children; ferritin; ferrous bisglycinate; hemoglobin; iron; iron deficiency; pregnancy; systematic review.
© The Author(s) 2023. Published by Oxford University Press on behalf of the International Life Sciences Institute. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




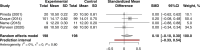


References
-
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–520. - PubMed
-
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control. Geneva, Switzerland: World Health Organization; 2001.
-
- Horton S, Ross J. The economics of iron deficiency. Food Policy. 2003;28:51–75.
-
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72:257S–264S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

