DEFINING "STRONG" VERSUS "WEAK" RESPONSE TO ANTIVASCULAR ENDOTHELIAL GROWTH FACTOR TREATMENT FOR CENTER-INVOLVED DIABETIC MACULAR EDEMA
- PMID: 36728692
- PMCID: PMC11040570
- DOI: 10.1097/IAE.0000000000003730
DEFINING "STRONG" VERSUS "WEAK" RESPONSE TO ANTIVASCULAR ENDOTHELIAL GROWTH FACTOR TREATMENT FOR CENTER-INVOLVED DIABETIC MACULAR EDEMA
Abstract
Background/purpose: To define "strong" versus "weak" antivascular endothelial growth factor (anti-VEGF) treatment response in eyes with center-involved diabetic macular edema (CI-DME).
Methods: Exploratory analyses of three DRCR Retina Network randomized trials of eyes with CI-DME treated with aflibercept, bevacizumab, or ranibizumab. Thresholds of 5-, 10-, and 15-letter gain defined strong visual acuity (VA) response when baseline VA was 20/25-20/32, 20/40-20/63, or 20/80-20/320, respectively. Thresholds of 50, 100, or 200- µ m reduction defined strong anatomical response when baseline central subfield thickness (CST) was <75, ≥75 to <175, or ≥175- µ m above standard thresholds. Additional thresholds from regression equations were calculated.
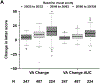
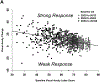
Results: At 24 weeks, outcomes for strong response were achieved by 476 of 958 eyes (50%) for VA and 505 eyes (53%) for CST. At 104 weeks among the 32% of eyes with strong VA and CST response at 24 weeks, 195 of 281 (69%) maintained strong VA and CST response, whereas 20 (7%) had neither strong VA nor strong CST response. Outcomes rates were similar across protocols and when defined using regression equations.
Conclusion: These phenotypes are suitable for efforts to identify predictive biomarkers for response to anti-VEGF therapy for DME and might facilitate comparison of treatment response among diverse cohorts with DME.
Figures








References
-
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. - PubMed
-
- Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(6):2247–2254. - PubMed
-
- Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114(10):1804–1809. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

