Discovery of novel 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives as multifunctional MAO-B inhibitors for the treatment of Parkinson's disease
- PMID: 36728713
- PMCID: PMC9897792
- DOI: 10.1080/14756366.2022.2159957
Discovery of novel 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives as multifunctional MAO-B inhibitors for the treatment of Parkinson's disease
Abstract
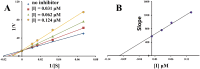
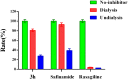
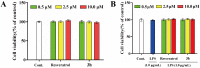
To discover novel multifunctional agents for the treatment of Parkinson's disease, a series of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives was designed, synthesized and evaluated. The results revealed that representative compound 3h possessed potent and selective MAO-B inhibitory activity (IC50 = 0.062 µM), and its inhibitory mode was competitive and reversible. Additionally, 3h also displayed excellent anti-oxidative effect (ORAC = 2.27 Trolox equivalent), significant metal chelating ability and appropriate BBB permeability. Moreover, 3h exhibited good neuroprotective effect and anti-neuroinflammtory ability. These results indicated that compound 3h was a promising candidate for further development against PD.
Keywords: 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives; MAO-B inhibitors; Parkinson’s disease; anti-neuroinflammatory agents; multifunctional anti-PD agents.
Conflict of interest statement
The authors report no conflicts of interest.
Figures







Similar articles
-
Discovery of novel 2-hydroxyl-4-benzyloxybenzyl aniline derivatives as potential multifunctional agents for the treatment of Parkinson's disease.Eur J Med Chem. 2023 Mar 5;249:115142. doi: 10.1016/j.ejmech.2023.115142. Epub 2023 Jan 21. Eur J Med Chem. 2023. PMID: 36716641
-
2-Hydroxy-4-benzyloxylimine Resveratrol Derivatives as Potential Multifunctional Agents for the Treatment of Parkinson's Disease.ChemMedChem. 2023 Mar 14;18(6):e202200629. doi: 10.1002/cmdc.202200629. Epub 2023 Jan 24. ChemMedChem. 2023. PMID: 36622947
-
Discovery of coumarin Mannich base derivatives as multifunctional agents against monoamine oxidase B and neuroinflammation for the treatment of Parkinson's disease.Eur J Med Chem. 2019 Jul 1;173:203-212. doi: 10.1016/j.ejmech.2019.04.016. Epub 2019 Apr 12. Eur J Med Chem. 2019. PMID: 31005056
-
Novel MAO-B inhibitors: potential therapeutic use of the selective MAO-B inhibitor PF9601N in Parkinson's disease.Int Rev Neurobiol. 2011;100:217-36. doi: 10.1016/B978-0-12-386467-3.00011-X. Int Rev Neurobiol. 2011. PMID: 21971010 Review.
-
Monoamine oxidase B inhibitors for the treatment of Parkinson's disease: a review of symptomatic and potential disease-modifying effects.CNS Drugs. 2011 Dec 1;25(12):1061-71. doi: 10.2165/11596310-000000000-00000. CNS Drugs. 2011. PMID: 22133327 Review.
Cited by
-
A Review on Therapeutic Strategies against Parkinson's Disease: Current Trends and Future Perspectives.Mini Rev Med Chem. 2025;25(2):96-111. doi: 10.2174/0113895575303788240606054620. Mini Rev Med Chem. 2025. PMID: 38918988 Review.
-
Molecular pathways: the quest for effective MAO-B inhibitors in neurodegenerative therapy.Mol Biol Rep. 2025 Feb 17;52(1):240. doi: 10.1007/s11033-025-10349-x. Mol Biol Rep. 2025. PMID: 39961877 Review.
-
The mechanism of ferroptosis and its related diseases.Mol Biomed. 2023 Oct 16;4(1):33. doi: 10.1186/s43556-023-00142-2. Mol Biomed. 2023. PMID: 37840106 Free PMC article. Review.
References
-
- Bloem BR, Okun MS, Klein C.. Parkinson’s disease. Lancet. 2021; 397(10291):2284–2303. - PubMed
-
- Hayes MT. Parkinson’s disease and Parkinsonism. Am J Med. 2019;132(7):802–807. - PubMed
-
- Dorsey ER, Elbaz A, Nichols E, Abbasi N, Abd-Allah F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi J-YJ, et al. . Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(11):939–953. - PMC - PubMed
-
- Azam F, El-Gnidi BA, Alkskas IA, Ahmed MA.. Design, synthesis and anti-Parkinsonian evaluation of 3-alkyl/aryl-8-(furan-2-yl)thiazolo[5,4-e][1,2,4]triazolo[1,5-c]pyrimidine-2(3H)-thiones against neuroleptic-induced catalepsy and oxidative stress in mice. J Enzyme Inhib Med Chem. 2010;25(6):818–826. - PubMed
-
- Chen Z, Li G, Liu J.. Autonomic dysfunction in Parkinson’s disease: implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 2020;134:104700. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
