Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23
- PMID: 36729117
- PMCID: PMC9896608
- DOI: 10.2807/1560-7917.ES.2023.28.5.2300043
Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23
Abstract
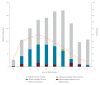
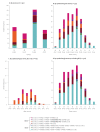
The Canadian Sentinel Practitioner Surveillance Network estimated vaccine effectiveness (VE) during the unusually early 2022/23 influenza A(H3N2) epidemic. Like vaccine, circulating viruses were clade 3C.2a1b.2a.2, but with genetic diversity affecting haemagglutinin positions 135 and 156, and reassortment such that H156 viruses acquired neuraminidase from clade 3C.2a1b.1a. Vaccine provided substantial protection with A(H3N2) VE of 54% (95% CI: 38 to 66) overall. VE was similar against H156 and vaccine-like S156 viruses, but with potential variation based on diversity at position 135.
Keywords: A(H3N2); Influenza; clade; imprinting; observational study; reassortment; test-negative design; vaccine effectiveness.
Conflict of interest statement
Figures
References
-
- Public Health Agency of Canada (PHAC). FluWatch report: January 8 to January 14, 2023 (week 2). Ottawa: PHAC; 2023. [Accessed: 31 Jan 2023). Available from: https://www.canada.ca/en/public-health/services/diseases/flu-influenza/i...
-
- European Centre for Disease Prevention and Control (ECDC). Flu New Europe: Joint ECDC-WHO/Europe weekly influenza update. Week 02/2023 (9 January – 15 January 2023). [Accessed: 31 Jan 2023]. Stockholm: ECDC; 2022. Available from: http://flunewseurope.org
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
