Targeting the vascular endothelial growth factor A/neuropilin 1 axis for relief of neuropathic pain
- PMID: 36729125
- PMCID: PMC10277229
- DOI: 10.1097/j.pain.0000000000002850
Targeting the vascular endothelial growth factor A/neuropilin 1 axis for relief of neuropathic pain
Abstract
Vascular endothelial growth factor A (VEGF-A) is a pronociceptive factor that causes neuronal sensitization and pain. We reported that blocking the interaction between the membrane receptor neuropilin 1 (NRP1) and VEGF-A-blocked VEGF-A-mediated sensory neuron hyperexcitability and reduced mechanical hypersensitivity in a rodent chronic neuropathic pain model. These findings identified the NRP1-VEGF-A signaling axis for therapeutic targeting of chronic pain. In an in-silico screening of approximately 480 K small molecules binding to the extracellular b1b2 pocket of NRP1, we identified 9 chemical series, with 6 compounds disrupting VEGF-A binding to NRP1. The small molecule with greatest efficacy, 4'-methyl-2'-morpholino-2-(phenylamino)-[4,5'-bipyrimidin]-6(1H)-one, designated NRP1-4, was selected for further evaluation. In cultured primary sensory neurons, VEGF-A enhanced excitability and decreased firing threshold, which was blocked by NRP1-4. In addition, NaV1.7 and CaV2.2 currents and membrane expression were potentiated by treatment with VEGF-A, and this potentiation was blocked by NRP1-4 cotreatment. Neuropilin 1-4 reduced VEGF-A-mediated increases in the frequency and amplitude of spontaneous excitatory postsynaptic currents in dorsal horn of the spinal cord. Neuropilin 1-4 did not bind to more than 300 G-protein-coupled receptors and receptors including human opioids receptors, indicating a favorable safety profile. In rats with spared nerve injury-induced neuropathic pain, intrathecal administration of NRP1-4 significantly attenuated mechanical allodynia. Intravenous treatment with NRP1-4 reversed both mechanical allodynia and thermal hyperalgesia in rats with L5/L6 spinal nerve ligation-induced neuropathic pain. Collectively, our findings show that NRP1-4 is a first-in-class compound targeting the NRP1-VEGF-A signaling axis to control voltage-gated ion channel function, neuronal excitability, and synaptic activity that curb chronic pain.
Copyright © 2023 International Association for the Study of Pain.
Figures

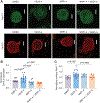
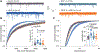

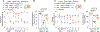
Comment in
-
From SARS-CoV-2 to analgesia: harnessing the vascular endothelial growth factor A/neuropilin 1 axis for pain therapy.Pain. 2023 Jul 1;164(7):1403-1405. doi: 10.1097/j.pain.0000000000002851. Epub 2022 Dec 15. Pain. 2023. PMID: 36651581 No abstract available.
References
-
- Amour FE, Smith DL. A METHOD FOR DETERMINING LOSS OF PAIN SENSATION. Journal of Pharmacology and Experimental Therapeutics 1941;72(1):74.
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33(1):87–107. - PubMed
-
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands to polypharmacological profiles. Nature 2012;492(7428):215–220. - PMC - PubMed
-
- Blevins JE, Stanley BG, Reidelberger RD. DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav 2002;71(1–2):277–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

