Short-term hepatocyte function and portal hypertension outcomes of sofosbuvir/velpatasvir for decompensated hepatitis C-related cirrhosis
- PMID: 36729172
- PMCID: PMC10049944
- DOI: 10.1007/s00535-023-01963-2
Short-term hepatocyte function and portal hypertension outcomes of sofosbuvir/velpatasvir for decompensated hepatitis C-related cirrhosis
Abstract
Background: It is unclear whether hepatocyte function and/or portal hypertension improves if a sustained virologic response (SVR) is achieved with direct-acting antivirals in patients with decompensated hepatitis C-related cirrhosis.
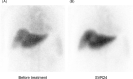
Methods: We examined the safety and efficacy of a 12-week course of sofosbuvir/velpatasvir (SOF/VEL) in 20 patients with decompensated hepatitis C-related cirrhosis. We also investigated changes in the hepatocyte receptor index (LHL15) and blood clearance index (HH15) by Tc-99 m-galactosyl human serum albumin scintigraphy, liver stiffness measurement (LSM) by transient elastography, and hepatic venous pressure gradient (HVPG) in patients who achieved an SVR at 24 weeks after treatment (SVR24).
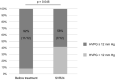
Results: One patient discontinued treatment because of rectal variceal hemorrhage, and 19 patients completed treatment. SVR24 was achieved in 17 patients (89%). Median LHL15 increased from 0.72 pre-treatment to 0.82 after SVR24 (p = 0.012), and median HH15 decreased from 0.82 pre-treatment to 0.76 after SVR24 (p = 0.010). The percentage of patients with LSM ≥ 20 kPa was 90% before treatment and remained at 90% after SVR24. However, the percentage with severe portal hypertension (defined as HVPG ≥ 12 mmHg) decreased from 92% pre-treatment to 58% after SVR24 (p = 0.046). Patients with a decreased HVPG from pre-treatment to after SVR24 had a smaller pre-treatment spleen volume than those with an increased HVPG (median, 252 vs. 537 mL, p = 0.028).
Conclusion: Achieving SVR24 with SOF/VEL treatment in patients with decompensated hepatitis C-related cirrhosis can be expected to improve hepatocyte function and portal hypertension on short-term follow-up.
Keywords: Hepatitis C; Liver cirrhosis; Portal hypertension; Sofosbuvir; Velpatasvir.
© 2023. The Author(s).
Conflict of interest statement
NK received lecture fees and research funding from Gilead Sciences, AbbVie, and MSD, and a scholarship donation from AbbVie. AT received research funding from Gilead Sciences. HF received research funding from MSD. SUK received research funding from Bristol-Myers and MSD, and scholarship donation from AbbVie. All other authors have no conflicts of interest to disclose.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

