Pharmacokinetic and Target Engagement Measures of ANX007, an Anti-C1q Antibody Fragment, Following Intravitreal Administration in Nonhuman Primates
- PMID: 36729444
- PMCID: PMC9907371
- DOI: 10.1167/iovs.64.2.3
Pharmacokinetic and Target Engagement Measures of ANX007, an Anti-C1q Antibody Fragment, Following Intravitreal Administration in Nonhuman Primates
Abstract
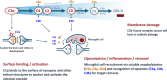
Purpose: C1q and the classical complement cascade are key regulators of synaptic pruning, and their aberrant activation has been implicated in neurodegenerative ophthalmic diseases including geographic atrophy and glaucoma. The antigen-binding fragment antibody ANX007 specifically recognizes globular head groups of C1q to block substrate binding and functionally inhibit classical complement cascade activation. ANX007 was assessed in nonclinical studies of biodistribution and C1q target engagement in the eye following intravitreal (IVT) administration in cynomolgus monkeys.
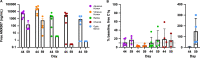
Methods: Female juvenile cynomolgus monkeys (n = 12) received a single bilateral dose of 1 or 5 mg ANX007/eye, with vitreous and non-perfused tissue samples collected approximately 4 weeks later. In a separate study, male (n = 6/5) and female (n = 6/5) animals received repeat bilateral dosing of 1, 2.5, or 5 mg ANX007/eye on days 1 and 29, with aqueous and vitreous collections on day 44 or day 59. Tissues from the 5 mg/eye repeat-dose group were perfused, and retina, choroid, and optic nerve samples were collected approximately 2 and 4 weeks post-last dose.
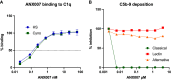
Results: Following a single dose of ANX007, vitreous levels of free drug were measurable through 4 weeks at both the 1 and 5 mg dose levels, with approximately 3-day half-life. With repeat dose of 5 mg/eye, free-ANX007 was measurable 4 weeks post-last dose in perfused retina and choroid and up to approximately 2 weeks post-last dose in optic nerve. There was a strong correlation between C1q target engagement and free drug levels in aqueous and vitreous humors and retinal tissue.
Conclusions: Following IVT administration, ANX007 distributes to sites within the retina that are relevant to neurodegenerative ophthalmic disease with clear evidence of C1q target engagement. Based on its mechanism of action inhibiting C1q and its downstream activity, ANX007 is predicted to mitigate tissue damage driven by classical complement activation in the retina. These data support further clinical evaluation of ANX007.
Conflict of interest statement
Disclosure:
Figures


References
-
- Stevens B, Allen NJ, Vazquez LE, et al. .. The classical complement cascade mediates CNS synapse elimination. Cell. 2007; 131: 1164–1178. - PubMed
-
- Stephan AH, Barres BA, Stevens B.. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012; 35: 369–389. - PubMed
-
- Stasi K, Nagel D, Yang X, et al. .. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci . 2006; 47(3): 1024–1029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

