Persistent binding at dopamine transporters determines sustained psychostimulant effects
- PMID: 36730201
- PMCID: PMC9963675
- DOI: 10.1073/pnas.2114204120
Persistent binding at dopamine transporters determines sustained psychostimulant effects
Abstract
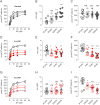
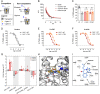
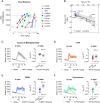
Psychostimulants interacting with the dopamine transporter (DAT) can be used illicitly or for the treatment of specific neuropsychiatric disorders. However, they can also produce severe and persistent adverse events. Often, their pharmacological properties in vitro do not fully correlate to their pharmacological profile in vivo. Here, we investigated the pharmacological effects of enantiomers of pyrovalerone, α-pyrrolidinovalerophenone, and 3,4-methylenedioxypyrovalerone as compared to the traditional psychostimulants cocaine and methylphenidate, using a variety of in vitro, computational, and in vivo approaches. We found that in vitro drug-binding kinetics at DAT correlate with the time-course of in vivo psychostimulant action in mice. In particular, a slow dissociation (i.e., slow koff) of S-enantiomers of pyrovalerone analogs from DAT predicts their more persistent in vivo effects when compared to cocaine and methylphenidate. Overall, our findings highlight the critical importance of drug-binding kinetics at DAT for determining the in vivo profile of effects produced by psychostimulant drugs.
Keywords: cathinones; dopamine transporter; drug-binding kinetics; new psychoactive substances; psychostimulants.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

