Impact of Chronotherapy on 6-Mercaptopurine Metabolites in Inflammatory Bowel Disease: A Pilot Crossover Trial
- PMID: 36730289
- PMCID: PMC9945554
- DOI: 10.14309/ctg.0000000000000549
Impact of Chronotherapy on 6-Mercaptopurine Metabolites in Inflammatory Bowel Disease: A Pilot Crossover Trial
Abstract
Introduction: Chronotherapy is the timing of medication according to biological rhythms of the host to optimize drug efficacy and minimize toxicity. Efficacy and myelosuppression of azathioprine/6-mercaptopurine (AZA/6-MP) are correlated with the metabolite 6-thioguanine, while the metabolite 6-methylmercaptopurine correlates with hepatotoxicity.
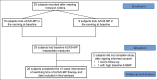
Methods: This was a single-center, 10-week prospective crossover trial involving 26 participants with inactive inflammatory bowel disease (IBD) on a stable dose and time of AZA or 6-MP therapy. Participants were switched to the opposite delivery time (morning or evening) for 10 weeks, and metabolite measurements were at both time points.
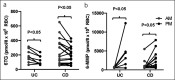
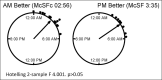
Results: In the morning vs evening dosing, 6-thioguanine levels were 225.7 ± 155.1 vs 175.0 ± 106.9 ( P < 0.01), and 6-methylmercaptopurine levels were 825.1 ± 1,023.3 vs 2,395.3 ± 2,880.3 ( P < 0.01), with 69% (18 out of 26) of participants had better metabolite profiles in the morning. Participants with optimal dosing in the morning had an earlier chronotype by corrected midpoint of sleep.
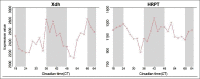
Discussion: In the first study on a potential role of chronotherapy in IBD, we found (i) morning dosing of AZA or 6-MP resulted in more optimal metabolite profiles and (ii) host chronotype could help identify one-third of patients who would benefit from evening dosing. Circadian regulation of metabolic enzymes of AZA/6-MP activity in the liver is the likely cause of these differences. This pilot study confirms the need to incorporate chronotherapy in future multicenter clinical trials on IBD disease.
Trial registration: ClinicalTrials.gov NCT04304950 NCT0430495..
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Abraham BP, Ahmed T, Ali T. Inflammatory bowel disease: Pathophysiology and current therapeutic approaches. Handb Exp Pharmacol 2017;239:115–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

