Biomechanics of keratoconus: Two numerical studies
- PMID: 36730305
- PMCID: PMC9894483
- DOI: 10.1371/journal.pone.0278455
Biomechanics of keratoconus: Two numerical studies
Abstract
Background: The steep cornea in keratoconus can greatly impair eyesight. The etiology of keratoconus remains unclear but early injury that weakens the corneal stromal architecture has been implicated. To explore keratoconus mechanics, we conducted two numerical simulation studies.
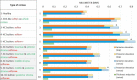
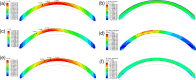
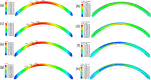
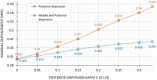
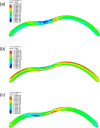
Methods: A finite-element model describing the five corneal layers and the heterogeneous mechanical behaviors of the ground substance and lamellar collagen-fiber architecture in the anterior and posterior stroma was developed using the Holzapfel-Gasser-Ogden constitutive model. The geometry was from a healthy subject. Its stroma was divided into anterior, middle, and posterior layers to assess the effect of changing regional mechanical parameters on corneal displacement and maximum principal stress under intraocular pressure. Specifically, the effect of softening an inferocentral corneal button, the collagen-based tissues throughout the whole cornea, or specific stromal layers in the button was examined. The effect of simply disorganizing the orthogonally-oriented posterior stromal fibers in the button was also assessed. The healthy cornea was also subjected to eye rubbing-like loading to identify the corneal layer(s) that experienced the most tensional stress.
Results: Conical deformation and corneal thinning emerged when the corneal button or the mid-posterior stroma of the button underwent gradual softening or when the collagen fibers in the mid-posterior stroma of the button were dispersed. Softening the anterior layers of the button or the whole cornea did not evoke conical deformation. Button softening greatly increased and disrupted the stress on Bowman's membrane while mid-posterior stromal softening increased stress in the anterior layers. Eye rubbing profoundly stressed the deep posterior stroma while other layers were negligibly affected.
Discussion: These observations suggest that keratoconus could be initiated, at least partly, by mechanical instability/damage in the mid-posterior stroma that then imposes stress on the anterior layers. This may explain why subclinical keratoconus is marked by posterior but not anterior elevation on videokeratoscopy.
Copyright: © 2023 Falgayrettes et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Ubels DL, Dawson DG, Edelhauser HF. Adler’s physiology of the eye. Elsevier, editor. 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

