Integrin-Dependent Cell-Matrix Adhesion in Endothelial Health and Disease
- PMID: 36730379
- PMCID: PMC9891302
- DOI: 10.1161/CIRCRESAHA.122.322332
Integrin-Dependent Cell-Matrix Adhesion in Endothelial Health and Disease
Abstract
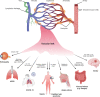
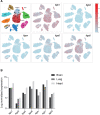
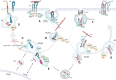
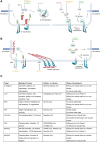
The endothelium is a dynamic, semipermeable layer lining all blood vessels, regulating blood vessel formation and barrier function. Proper composition and function of the endothelial barrier are required for fluid homeostasis, and clinical conditions characterized by barrier disruption are associated with severe morbidity and high mortality rates. Endothelial barrier properties are regulated by cell-cell junctions and intracellular signaling pathways governing the cytoskeleton, but recent insights indicate an increasingly important role for integrin-mediated cell-matrix adhesion and signaling in endothelial barrier regulation. Here, we discuss diseases characterized by endothelial barrier disruption, and provide an overview of the composition of endothelial cell-matrix adhesion complexes and associated signaling pathways, their crosstalk with cell-cell junctions, and with other receptors. We further present recent insights into the role of cell-matrix adhesions in the developing and mature/adult endothelium of various vascular beds, and discuss how the dynamic regulation and turnover of cell-matrix adhesions regulates endothelial barrier function in (patho)physiological conditions like angiogenesis, inflammation and in response to hemodynamic stress. Finally, as clinical conditions associated with vascular leak still lack direct treatment, we focus on how understanding of endothelial cell-matrix adhesion may provide novel targets for treatment, and discuss current translational challenges and future perspectives.
Keywords: angiogenesis; cell-matrix adhesions; edema; endothelium; inflammation; integrins.
Figures



References
-
- Shyy JYJ, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

