Delivery of small molecule mast cell activators for West Nile Virus vaccination using acetalated dextran microparticles
- PMID: 36731641
- PMCID: PMC9975031
- DOI: 10.1016/j.ijpharm.2023.122658
Delivery of small molecule mast cell activators for West Nile Virus vaccination using acetalated dextran microparticles
Abstract
Recently, there has been increasing interest in the activation of mast cells to promote vaccine efficacy. Several mast cell activating (MCA) compounds have been reported such as M7 and Compound 48/80 (C48/80). While these MCAs have been proven to be efficacious vaccine adjuvants, their translatability is limited by batch-to-batch variability, challenging large-scale manufacturing, and poor in vivo stability for the M7 peptide. Due to this, high throughput screening was performed to identify small molecule MCAs. Several potent MCAs were identified via this screening, but the in vivo translatability of the compounds was limited due to their poor aqueous solubility. To enhance the delivery of these MCAs we encapsulated them in acetalated dextran (Ace-DEX) microparticles (MPs). We have previously utilized Ace-DEX MPs for vaccine delivery due to their passive targeting to phagocytic cells, acid sensitivity, and tunable degradation. Four different MCA loaded MPs were combined with West Nile Virus Envelope III protein (EDIII) and their vaccine adjuvant activities were compared in vivo. MPs containing the small molecule MCA ST101036 produced the highest anti-EDIII IgG titers of all the MCAs tested. Further, ST101036 MPs produced higher titers than ST101036 formulated with PEG as a cosolvent which highlights the benefit of Ace-DEX MPs over a conventional formulation technique. Finally, in a mouse model of West Nile Virus infection ST101036 MPs produced similar survival to soluble M7 (80-90%). Overall, these data show that ST101036 MPs produce a robust antibody response against EDIII and survival emphasizing the benefits of using Ace-DEX as a delivery platform for the poorly soluble ST101036.
Keywords: Acetalated dextran; Mast cell activator; Microparticles; Vaccine; West Nile Virus.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ainslie reports financial support was provided by National Institutes of Health. Bachelder has patent #PCT/US2009/049415 pending to BERKELEY LAB.
Figures

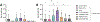
Similar articles
-
Nasal Immunization With Small Molecule Mast Cell Activators Enhance Immunity to Co-Administered Subunit Immunogens.Front Immunol. 2021 Sep 10;12:730346. doi: 10.3389/fimmu.2021.730346. eCollection 2021. Front Immunol. 2021. PMID: 34566991 Free PMC article.
-
Mast cell activators as adjuvants for intranasal mucosal vaccines.Int J Pharm. 2025 Mar 15;672:125300. doi: 10.1016/j.ijpharm.2025.125300. Epub 2025 Feb 4. Int J Pharm. 2025. PMID: 39914508 Review.
-
Tunable degradation of acetalated dextran microparticles enables controlled vaccine adjuvant and antigen delivery to modulate adaptive immune responses.J Control Release. 2018 Mar 10;273:147-159. doi: 10.1016/j.jconrel.2018.01.027. Epub 2018 Feb 2. J Control Release. 2018. PMID: 29407676 Free PMC article.
-
In Vivo and Cellular Trafficking of Acetalated Dextran Microparticles for Delivery of a Host-Directed Therapy for Salmonella enterica Serovar Typhi Infection.Mol Pharm. 2018 Nov 5;15(11):5336-5348. doi: 10.1021/acs.molpharmaceut.8b00802. Epub 2018 Oct 17. Mol Pharm. 2018. PMID: 30296381 Free PMC article.
-
Acetalated dextran based nano- and microparticles: synthesis, fabrication, and therapeutic applications.Chem Commun (Camb). 2021 Apr 29;57(35):4212-4229. doi: 10.1039/d1cc00811k. Chem Commun (Camb). 2021. PMID: 33913978 Review.
Cited by
-
West Nile Virus in a changing climate: epidemiology, pathology, advances in diagnosis and treatment, vaccine designing and control strategies, emerging public health challenges - a comprehensive review.Emerg Microbes Infect. 2025 Dec;14(1):2437244. doi: 10.1080/22221751.2024.2437244. Epub 2025 Jan 2. Emerg Microbes Infect. 2025. PMID: 39614679 Free PMC article. Review.
-
Flash nanoprecipitation allows easy fabrication of pH-responsive acetalated dextran nanoparticles for intracellular release of payloads.Discov Nano. 2024 Jan 4;19(1):4. doi: 10.1186/s11671-023-03947-w. Discov Nano. 2024. PMID: 38175336 Free PMC article.
-
Ethoxy Acetalated Dextran-Based Biomaterials for Therapeutic Applications.Polymers (Basel). 2024 Sep 29;16(19):2756. doi: 10.3390/polym16192756. Polymers (Basel). 2024. PMID: 39408467 Free PMC article. Review.
References
-
- Bachelder EM, Pino EN, Ainslie KM, 2017. Acetalated Dextran: A Tunable and Acid-Labile Biopolymer with Facile Synthesis and a Range of Applications. American Chemical Society, pp. 1915–1926. - PubMed
-
- Batty CJ, Amouzougan EA, Carlock MA, Ross TM, Bachelder EM, Ainslie KM, 2022. Sustained delivery of CpG oligodeoxynucleotide by acetalated dextran microparticles augments effector response to Computationally Optimized Broadly Reactive Antigen (COBRA) influenza hemagglutinin. International Journal of Pharmaceutics. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

