Why falsified medicines reach patients: an analysis of political and economic factors in Romania
- PMID: 36731920
- PMCID: PMC10175937
- DOI: 10.1136/bmjgh-2022-009918
Why falsified medicines reach patients: an analysis of political and economic factors in Romania
Abstract
Introduction: To protect patients against falsified medicines, countries around the world implement stringent regulations. Despite efforts to protect supply chains in the European Union (EU), authorities continue to find falsified medicine. We studied how in Romania, one of the poorest EU countries, political and economic factors influence the risk of patients being exposed to falsified medicines.
Methods: For this case study, we reviewed 131 documents and interviewed 22 purposively selected key informants.
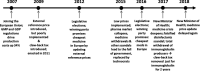
Results: In Romania, several politically and economically motivated policies have led to persistent medicine shortages. Following the 2007 accession to the EU, fierce competition led to a decline in domestic medicine production. Soon after, the government introduced a tax on reimbursed medicines to support the national health budget. Prior to the 2015 elections, medicine prices were abruptly lowered to provide voters with the cheapest medicine in Europe. The low prices incentivised traders to buy medicines in Romania and sell them elsewhere in the EU. The high taxes and low prices led manufacturers to withdraw medicines from the market and impose product quotas to limit parallel trading. The accumulated effect of these market responses translated into persistent shortages of essential medicine, which have pushed patients and health professionals to unregulated markets with a high risk of exposure to falsified medicine.
Conclusion: Strategies against falsified medicine with a narrow focus on safeguarding quality in the regulated supply are insufficient. To protect patients, governments must also ensure that patients have access to affordable medicines, as shortages provide an opportunity for those selling fake products.
Keywords: Health policy; Health services research; Health systems; Pharmacology; Public Health.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization . Who global surveillance and monitoring system for substandard and falsified medical products. Geneva, 2017. http://www.who.int/medicines/regulation/ssffc/publications/GSMS_Report.pdf
-
- European Commission . Directive 2011/62/EU of the European Parliament and of the Council, of 8 June 2011 amending Directive 2001/83/EC on the community code relating to medicinal products for human use, as regards the prevention of the entry into the legal supply chain of falsified medicinal products, 2011. Available: /health/human-use/falsified_medicines_en [Accessed 21 Jan 2018].
-
- Buckley GJB, Gostin LO. Countering the problem of falsified and substandard drugs: committee on understanding the global public health implications of substandard, falsified, and counterfeit medical products. Washington, D.C: National Academies Press, Institute of Medicine of the National Academies, 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous