Acute kidney injury and delayed methotrexate clearance in an adult patient with Philadelphia chromosome-positive acute lymphoblastic leukaemia treated with imatinib
- PMID: 36732034
- PMCID: PMC10447946
- DOI: 10.1136/ejhpharm-2022-003453
Acute kidney injury and delayed methotrexate clearance in an adult patient with Philadelphia chromosome-positive acute lymphoblastic leukaemia treated with imatinib
Abstract
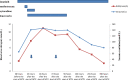
A female patient in her early 30s was treated with imatinib and high-dose methotrexate (HD-MTX) for Philadelphia chromosome-positive acute lymphoblastic leukaemia. The patient developed delayed MTX clearance and grade 3 acute kidney injury characterised by elevated creatinine (114% increase from baseline). After intensified calcium folinate rescue therapy and hydration, the MTX serum level was appropriately decreased 72 hours after the start of MTX infusion, and renal function returned to normal. Medication analysis by a clinical pharmacist suggested that the concomitant treatment with imatinib likely contributed to the delayed MTX clearance and caused the acute kidney injury.
Keywords: Acute Kidney Injury; DRUG-RELATED SIDE EFFECTS AND ADVERSE REACTIONS; Drug Monitoring; Leukemia; PHARMACY SERVICE, HOSPITAL; Safety.
© European Association of Hospital Pharmacists 2023. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Concurrent Imatinib Dosing With High-dose Methotrexate Leads to Acute Kidney Injury and Delayed Methotrexate Clearance in Pediatric Patients With Philadelphia Chromosome-positive B-Cell Acute Lymphoblastic Leukemia.J Pediatr Hematol Oncol. 2021 Mar 1;43(2):e296-e300. doi: 10.1097/MPH.0000000000001816. J Pediatr Hematol Oncol. 2021. PMID: 32398599
-
Delayed elimination of high-dose methotrexate and use of carboxypeptidase G2 in pediatric patients during treatment for acute lymphoblastic leukemia.Pediatr Blood Cancer. 2017 Jul;64(7). doi: 10.1002/pbc.26395. Epub 2016 Dec 14. Pediatr Blood Cancer. 2017. PMID: 27966809
-
Impact of pre-hydration duration on high-dose methotrexate induced nephrotoxicity in childhood acute lymphoblastic leukaemia in resource constraint centers: a randomized crossover study.Cancer Chemother Pharmacol. 2023 Apr;91(4):331-336. doi: 10.1007/s00280-023-04525-8. Epub 2023 Mar 23. Cancer Chemother Pharmacol. 2023. PMID: 36951972 Clinical Trial.
-
Imatinib: in relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukaemia.Drugs. 2007;67(17):2645-54. doi: 10.2165/00003495-200767170-00013. Drugs. 2007. PMID: 18034597 Review.
-
Glucarpidase treatment for methotrexate intoxication: a case report and review of the literature.Neth J Med. 2018 Jan;76(1):36-39. Neth J Med. 2018. PMID: 29380731 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources