Dose escalation randomised study of efmarodocokin alfa in healthy volunteers and patients with ulcerative colitis
- PMID: 36732049
- PMCID: PMC10359578
- DOI: 10.1136/gutjnl-2022-328387
Dose escalation randomised study of efmarodocokin alfa in healthy volunteers and patients with ulcerative colitis
Abstract
Background: The interleukin-22 cytokine (IL-22) has demonstrated efficacy in preclinical colitis models with non-immunosuppressive mechanism of action. Efmarodocokin alfa (UTTR1147A) is a fusion protein agonist that links IL-22 to the crystallisable fragment (Fc) of human IgG4 for improved pharmacokinetic characteristics, but with a mutation to minimise Fc effector functions.
Methods: This randomised, phase 1b study evaluated the safety, tolerability, pharmacokinetics and pharmacodynamics of repeat intravenous dosing of efmarodocokin alfa in healthy volunteers (HVs; n=32) and patients with ulcerative colitis (n=24) at 30-90 µg/kg doses given once every 2 weeks or monthly (every 4 weeks) for 12 weeks (6:2 active:placebo per cohort).
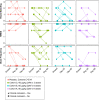
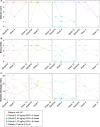
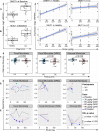
Results: The most common adverse events (AEs) were on-target, reversible, dermatological effects (dry skin, erythema and pruritus). Dose-limiting non-serious dermatological AEs (severe dry skin, erythema, exfoliation and discomfort) were seen at 90 μg/kg once every 2 weeks (HVs, n=2; patients, n=1). Pharmacokinetics were generally dose-proportional across the dose levels, but patients demonstrated lower drug exposures relative to HVs at the same dose. IL-22 serum biomarkers and IL-22-responsive genes in colon biopsies were induced with active treatment, and microbiota composition changed consistent with a reversal in baseline dysbiosis. As a phase 1b study, efficacy endpoints were exploratory only. Clinical response was observed in 7/18 active-treated and 1/6 placebo-treated patients; clinical remission was observed in 5/18 active-treated and 0/6 placebo-treated patients.
Conclusion: Efmarodocokin alfa had an adequate safety and pharmacokinetic profile in HVs and patients. Biomarker data confirmed IL-22R pathway activation in the colonic epithelium. Results support further investigation of this non-immunosuppressive potential inflammatory bowel disease therapeutic.
Trial registration number: NCT02749630.
Keywords: gene expression; interleukins; intestinal microbiology; ulcerative colitis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ANL, YW, MK, AD, BB, BH, LDO, JSM, HC and MER are employees and stockholders of Genentech/Roche.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
