Deletion of SNX9 alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy
- PMID: 36732507
- PMCID: PMC9895440
- DOI: 10.1038/s41467-022-35583-w
Deletion of SNX9 alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy
Abstract
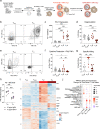
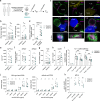
Tumor-specific T cells are frequently exhausted by chronic antigenic stimulation. We here report on a human antigen-specific ex vivo model to explore new therapeutic options for T cell immunotherapies. T cells generated with this model resemble tumor-infiltrating exhausted T cells on a phenotypic and transcriptional level. Using a targeted pooled CRISPR-Cas9 screen and individual gene knockout validation experiments, we uncover sorting nexin-9 (SNX9) as a mediator of T cell exhaustion. Upon TCR/CD28 stimulation, deletion of SNX9 in CD8 T cells decreases PLCγ1, Ca2+, and NFATc2-mediated T cell signaling and reduces expression of NR4A1/3 and TOX. SNX9 knockout enhances memory differentiation and IFNγ secretion of adoptively transferred T cells and results in improved anti-tumor efficacy of human chimeric antigen receptor T cells in vivo. Our findings highlight that targeting SNX9 is a strategy to prevent T cell exhaustion and enhance anti-tumor immunity.
© 2023. The Author(s).
Conflict of interest statement
H.L., A.Zippelius received research funding from Bristol-Myers Squibb and Novartis. A.Zippelius received consulting/advisor fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Hoffmann–La Roche, NBE Therapeutics, Secarna, ACM Pharma, and Hookipa, and maintains further non-commercial research agreements with Secarna, Hookipa, Roche and Beyondsprings. L.T.J. is a co-founder of, holds equity in and has a sponsored research agreement with Cimeio Therapeutics AG. A.Zippelius and M.P.T. have a pending patent application (PCT/EP2022/077391, “Targeting SNX9 rescues recombinant T cell in adoptive therapy”). The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

