3-Mercaptopyruvate sulfur transferase is a protein persulfidase
- PMID: 36732619
- PMCID: PMC10060159
- DOI: 10.1038/s41589-022-01244-8
3-Mercaptopyruvate sulfur transferase is a protein persulfidase
Abstract
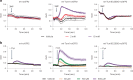
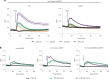
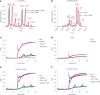
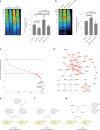
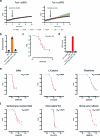
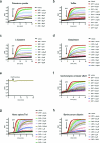
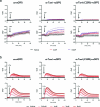
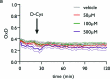
Protein S-persulfidation (P-SSH) is recognized as a common posttranslational modification. It occurs under basal conditions and is often observed to be elevated under stress conditions. However, the mechanism(s) by which proteins are persulfidated inside cells have remained unclear. Here we report that 3-mercaptopyruvate sulfur transferase (MPST) engages in direct protein-to-protein transpersulfidation reactions beyond its previously known protein substrates thioredoxin and MOCS3/Uba4, associated with H2S generation and transfer RNA thiolation, respectively. We observe that depletion of MPST in human cells lowers overall intracellular protein persulfidation levels and identify a subset of proteins whose persulfidation depends on MPST. The predicted involvement of these proteins in the adaptation to stress responses supports the notion that MPST-dependent protein persulfidation promotes cytoprotective functions. The observation of MPST-independent protein persulfidation suggests that other protein persulfidases remain to be identified.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

