First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial
- PMID: 36732627
- PMCID: PMC9941045
- DOI: 10.1038/s41591-022-02179-2
First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial
Abstract
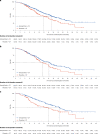
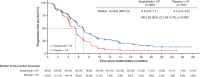
First-line systemic therapeutic options for advanced esophageal squamous cell carcinoma (ESCC) are limited. In this multicenter, double-blind phase 3 trial, a total of 551 patients with previously untreated, locally advanced or metastatic ESCC and PD-L1 combined positive score of ≥1 were randomized (2:1) to receive serplulimab (an anti-PD-1 antibody; 3 mg/kg) or placebo (on day 1), plus cisplatin (50 mg/m2) (on day 1) and continuous infusion of 5-fluorouracil (1,200 mg/m2) (on days 1 and 2), once every 2 weeks. The study met the primary endpoints. At the prespecified final analysis of progression-free survival (PFS) assessed by the blinded independent radiological review committee, serplulimab plus chemotherapy significantly improved PFS compared with placebo plus chemotherapy (median PFS of 5.8 months and 5.3 months, respectively; hazard ratio, 0.60; 95% confidence interval, 0.48-0.75; P < 0.0001). At the prespecified interim analysis of overall survival (OS), serplulimab plus chemotherapy also significantly prolonged OS compared with placebo plus chemotherapy (median OS of 15.3 months and 11.8 months, respectively; hazard ratio, 0.68; 95% confidence interval, 0.53-0.87; P = 0.0020). Grade 3 or higher treatment-related adverse events occurred in 201 (53%) and 81 (48%) patients in the serplulimab plus chemotherapy group and the placebo plus chemotherapy group, respectively. Serplulimab plus chemotherapy administered every 2 weeks significantly improved PFS and OS in patients with previously untreated, PD-L1-positive advanced ESCC, with a manageable safety profile. This study is registered with ClinicalTrials.gov ( NCT03958890 ).
© 2023. The Author(s).
Conflict of interest statement
Y.K., J.L., Q.W. and J.Z. are employees of Shanghai Henlius Biotech, Inc. J.H. has served a consulting or advisory role for Merck Sharp & Dohme Oncology and Roche. All other authors declare no competing interests.
Figures


References
-
- Luo H, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–925. doi: 10.1001/jama.2021.12836. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

