Common mouse models of tauopathy reflect early but not late human disease
- PMID: 36732784
- PMCID: PMC9893608
- DOI: 10.1186/s13024-023-00601-y
Common mouse models of tauopathy reflect early but not late human disease
Abstract
Background: Mouse models that overexpress human mutant Tau (P301S and P301L) are commonly used in preclinical studies of Alzheimer's Disease (AD) and while several drugs showed therapeutic effects in these mice, they were ineffective in humans. This leads to the question to which extent the murine models reflect human Tau pathology on the molecular level.
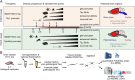
Methods: We isolated insoluble, aggregated Tau species from two common AD mouse models during different stages of disease and characterized the modification landscape of the aggregated Tau using targeted and untargeted mass spectrometry-based proteomics. The results were compared to human AD and to human patients that suffered from early onset dementia and that carry the P301L Tau mutation.
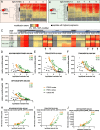
Results: Both mouse models accumulate insoluble Tau species during disease. The Tau aggregation is driven by progressive phosphorylation within the proline rich domain and the C-terminus of the protein. This is reflective of early disease stages of human AD and of the pathology of dementia patients carrying the P301L Tau mutation. However, Tau ubiquitination and acetylation, which are important to late-stage human AD are not represented in the mouse models.
Conclusion: AD mouse models that overexpress human Tau using risk mutations are a suitable tool for testing drug candidates that aim to intervene in the early formation of insoluble Tau species promoted by increased phosphorylation of Tau.
Keywords: Alzheimer’s disease; Disease progression; Human Tau; Mouse model; P301L; P301S; Post-translational modifications; Protein aggregation; Quantitative proteomics; Tauopathy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

