Toll-like receptor 4 signaling pathway in sensory neurons mediates remifentanil-induced postoperative hyperalgesia via transient receptor potential ankyrin 1
- PMID: 36733260
- PMCID: PMC9926008
- DOI: 10.1177/17448069231158290
Toll-like receptor 4 signaling pathway in sensory neurons mediates remifentanil-induced postoperative hyperalgesia via transient receptor potential ankyrin 1
Abstract
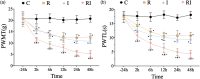
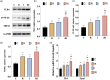
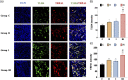
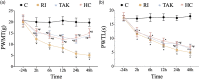
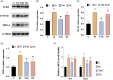
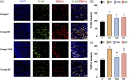
Background: Remifentanil-induced postoperative hyperalgesia (RIH) refers to a state of hyperalgesia or aggravated pre-existing pain after remifentanil exposure. There has been considerable interest in understanding and preventing RIH. However, the mechanisms responsible for RIH are still not completely understood. Toll-like receptor 4 (TLR4), a classic innate immune receptor, has been detected in sensory neurons and participates in various nociceptive conditions, whereas its role in RIH remains unclear. Transient receptor potential ankyrin 1 (TRPA1) always serves as a nociceptive channel, whereas its role in RIH has not yet been investigated. This study aimed to determine whether the TLR4 signaling pathway in sensory neurons engaged in the development of RIH and the possible involvement of TRPA1 during this process. Methods: A rat model of remifentanil-induced postoperative hyperalgesia (RIH) was established, which presented decreased paw withdrawal mechanical threshold (PWMT) and paw withdrawal thermal latency (PWTL). The mRNA and protein expression levels of TLR4, phosphorylated NF-κB, and TRPA1 in the dorsal root ganglion (DRG) from RIH model were analyzed by real-time PCR, western blot, and immunofluorescence. The TLR4 antagonist TAK-242 and the TRPA1 antagonist HC-030031 were applied to determine the role of sensory neuron TLR4 signaling and TRPA1 in RIH. Results: Compared with control, PWMT and PWTL were significantly decreased in RIH model. Moreover, the mRNA and protein expression of TLR4 and TRPA1 in DRG were upregulated after remifentanil exposure together with increased NF-κB phosphorylation. TLR4 antagonist TAK-242 mitigated mechanical pain in RIH together with downregulated expression of TLR4, phosphorylated NF-κB, and TRPA1 in DRG neurons. In addition, TRPA1 antagonist HC-030031 also alleviated mechanical pain and decreased TRPA1 expression in RIH without affecting TLR4 signaling in DRG. Conclusions: Taken together, these results suggested that activation of TLR4 signaling pathway engaged in the development of RIH by regulating TRPA1 in DRG neurons. Blocking TLR4 and TRPA1 might serve as a promising therapeutic strategy for RIH.
Keywords: Remifentanil-induced postoperative hyperalgesia; dorsal root ganglion; opioids; toll-like receptor 4 signaling; transient receptor potential ankyrin 1.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet (London, England) 2019; 393(10180): 1558–1568. - PubMed
-
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011; 14(2): 145–161. - PubMed
-
- Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth 2019; 122(6): e114–e126. - PubMed
-
- Kim Y, Bae H, Yoo S, Park SK, Lim YJ, Sakura S, Kim JT. Effect of remifentanil on postoperative analgesic consumption in patients undergoing shoulder arthroplasty after interscalene brachial plexus block: a randomized controlled trial. J Anesth 2022; 36(4): 506–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

