HER2 status in recurrent/metastatic androgen receptor overexpressing salivary gland carcinoma patients
- PMID: 36733354
- PMCID: PMC9887140
- DOI: 10.3389/fonc.2022.1096068
HER2 status in recurrent/metastatic androgen receptor overexpressing salivary gland carcinoma patients
Abstract
Background: Overexpression of human epidermal growth factor receptor type 2 (HER2) occurs in almost 25-30% of androgen receptor (AR)-positive salivary gland carcinomas (SGCs), notably salivary duct carcinoma (SDC) and adenocarcinoma not otherwise specified (NOS). In the last years, several studies have reported the clinical benefit of HER2 directed therapies in this setting. This work aims at describing the natural history of AR-positive recurrent/metastatic (R/M) SGC patients, based on HER2 amplification status.
Methods: Consecutive R/M AR-positive SGC patients accessing our Institution from 2010 to 2021 were analyzed. Descriptive statistics and survival analyses were performed to present the clinical characteristics of the selected patients and the outcomes, based on HER2 status. A specific focus was dedicated to patients developing metastases to the central nervous system (CNS).
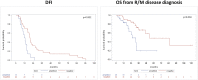
Results: Seventy-four R/M AR-positive SGC patients (72 men) were analyzed. Median follow-up was 36.18 months (95% CI 30.19-42.66). HER2 status was available in 62 cases (84%) and in 42% the protein was overexpressed (HER2+). Compared with patients with HER2- SGCs, in patients with HER2+ disease, HR for disease recurrence was 2.97 (95% CI 1.44-6.1, p=0.003), and HR for death from R/M disease was 3.22 (95% CI 1.39-7.49, p=0.007). Moreover, the HER2+ group showed a non-significant trend towards a higher prevalence of CNS metastases (40% vs. 24%, p=0.263). Patients developing CNS metastases had shorter survival than those who did not; at bivariate analysis (covariates: CNS disease and HER2 status), HER2 status demonstrated its independent prognostic significance.
Discussion: In our patient population, HER2 amplification was a negative prognostic factor, and it was associated with a non-statistically significant higher risk of developing CNS metastasis. Further studies are needed to explore the potential clinical benefit of tackling the two biological pathways (AR and HER2) in patients affected by this rare and aggressive malignancy.
Keywords: HER2; SDC; SGC; androgen receptor; brain metastasis; salivary duct carcinoma; salivary gland carcinoma.
Copyright © 2023 Cavalieri, Nuzzolese, Ottini, Bergamini, Resteghini, Colombo, Alfieri, Quattrone, Calareso, Iacovelli, Franceschini and Licitra.
Conflict of interest statement
LL declares the following conflicts of interest: research funds donated directly to the institute for clinical trials from Astrazeneca, BMS, Boehringer Ingelheim, Celgene International, Eisai, Exelixis, Debiopharm International SA, Hoffmann-La Roche ltd, IRX Therapeutics, Medpace, Merck-Serono, MSD, Novartis, Pfizer, Roche, Buran; occasional fees for participation as a speaker at conferences/congresses or as a scientific consultant for advisory boards from Astrazeneca, Bayer, MSD, Merck-Serono, AccMed, Neutron Therapeutics, Inc. CR declares honoraria from SunPharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. 4th ed. Fourth edi. Lyon, France: WHO - IARC; (2017).
-
- Bishop JA, Thompson LDR, Wakely PEJ, Weinreb I. AFIP atlas: tumors of the salivary glands (fifth series, vol. 5). ARP Press; Arlington, Virginia, USA (2021).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous