Temporal metabolic profiling of bone healing in a caprine tibia segmental defect model
- PMID: 36733424
- PMCID: PMC9886884
- DOI: 10.3389/fvets.2022.1023650
Temporal metabolic profiling of bone healing in a caprine tibia segmental defect model
Abstract
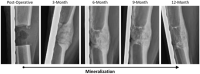
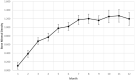
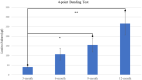
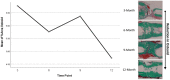
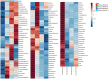
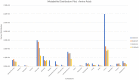
Bone tissue engineering is an emerging field of regenerative medicine, with a wide array of biomaterial technologies and therapeutics employed. However, it is difficult to objectively compare these various treatments during various stages of tissue response. Metabolomics is rapidly emerging as a powerful analytical tool to establish broad-spectrum metabolic signatures for a target biological system. Developing an effective biomarker panel for bone repair from small molecule data would provide an objective metric to readily assess the efficacy of novel therapeutics in relation to natural healing mechanisms. In this study we utilized a large segmental bone defect in goats to reflect trauma resulting in substantial volumetric bone loss. Characterization of the native repair capacity was then conducted over a period of 12 months through the combination of standard (radiography, computed tomography, histology, biomechanics) data and ultra-high-performance liquid chromatography-high resolution mass spectrometry (UHPLC-HRMS) metabolic profiling. Standard metrics demonstrated that samples formed soft callus structures that later mineralized. Small molecule profiles showed distinct temporal patterns associated with the bone tissue repair process. Specifically, increased lactate and amino acid levels at early time points indicated an environment conducive to osteoblast differentiation and extracellular matrix formation. Citrate and pyruvate abundances increased at later time points indicating increasing mineral content within the defect region. Taurine, shikimate, and pantothenate distribution profiles appeared to represent a shift toward a more homeostatic remodeling environment with the differentiation and activity of osteoclasts offsetting the earlier deposition phases of bone repair. The generation of a comprehensive metabolic reference portfolio offers a potent mechanism for examining novel biomaterials and can serve as guide for the development of new targeted therapeutics to improve the rate, magnitude, and quality of bone regeneration.
Keywords: biomarkers; bone remodeling; bone tissue engineering; caprine; large animal model; metabolomics; regenerative medicine; veterinary research.
Copyright © 2023 Bow, Rifkin, Priester, Christopher, Grzeskowiak, Hecht, Adair, Mulon, Castro, Campagna and Anderson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

