Ultrahigh-speed multimodal adaptive optics system for microscopic structural and functional imaging of the human retina
- PMID: 36733751
- PMCID: PMC9872887
- DOI: 10.1364/BOE.462594
Ultrahigh-speed multimodal adaptive optics system for microscopic structural and functional imaging of the human retina
Abstract
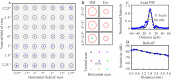
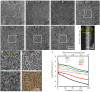
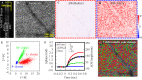
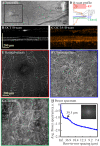
We describe the design and performance of a multimodal and multifunctional adaptive optics (AO) system that combines scanning laser ophthalmoscopy (SLO) and optical coherence tomography (OCT) for simultaneous retinal imaging at 13.4 Hz. The high-speed AO-OCT channel uses a 3.4 MHz Fourier-domain mode-locked (FDML) swept source. The system achieves exquisite resolution and sensitivity for pan-macular and transretinal visualization of retinal cells and structures while providing a functional assessment of the cone photoreceptors. The ultra-high speed also enables wide-field scans for clinical usability and angiography for vascular visualization. The FDA FDML-AO system is a powerful platform for studying various retinal and neurological diseases for vision science research, retina physiology investigation, and biomarker development.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the U.S. Department of Health and Human Services.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
