Micro-fragmented and nanofat adipose tissue derivatives: In vitro qualitative and quantitative analysis
- PMID: 36733959
- PMCID: PMC9887143
- DOI: 10.3389/fbioe.2023.911600
Micro-fragmented and nanofat adipose tissue derivatives: In vitro qualitative and quantitative analysis
Abstract
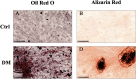
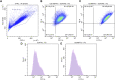
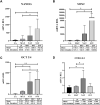
Introduction: Adipose tissue is widely exploited in regenerative medicine thanks to its trophic properties, mainly based on the presence of adipose-derived stromal cells. Numerous devices have been developed to promote its clinical use, leading to the introduction of one-step surgical procedures to obtain minimally manipulated adipose tissue derivatives. However, only a few studies compared their biological properties. This study aimed to characterize micro-fragmented (MAT) and nanofat adipose tissue (NAT) obtained with two different techniques. Methods: MAT, NAT and unprocessed lipoaspirate were collected from surgical specimens. RNA extraction and collagenase isolation of stromal vascular fraction (SVF) were performed. Tissue sections were analysed by histological and immunohistochemical (collagen type I, CD31, CD34 and PCNA) staining to assess tissue morphology and cell content. qPCR was performed to evaluate the expression of stemness-related (SOX2, NANOG and OCT3/4), extracellular matrix (COL1A1) and inflammatory genes (IL1β, IL6 and iNOS). Furthermore, multilineage differentiation was assessed following culture in adipogenic and osteogenic media and staining with Oil Red O and Alizarin red. ASC immunophenotype was assessed by flow cytometric analysis of CD90, CD105, CD73 and CD45. Results: Histological and immunohistochemical results showed an increased amount of stroma and a reduction of adipocytes in MAT and NAT, with the latter displaying the highest content of collagen type I, CD31, CD34 and PCNA. From LA to MAT and NAT, an increasing expression of NANOG, SOX2, OCT3/4, COL1A1 and IL6 was noted, while no significant differences in terms of IL1β and iNOS emerged. No statistically significant differences were noted between NAT and SVF in terms of stemness-related genes, while the latter demonstrated a significantly higher expression of stress-related markers. SVF cells derived from all three samples (LA, MAT, and NAT) showed a similar ASC immunoprofile as well as osteogenic and adipogenic differentiation. Discussion: Our results showed that both MAT and NAT techniques allowed the rapid isolation of ASC-rich grafts with a high anabolic and proliferative potential. However, NAT showed the highest levels of extracellular matrix content, replicating cells, and stemness gene expression. These results may provide precious clues for the use of adipose tissue derivatives in the clinical setting.
Keywords: adipose tissue; cell therapy; mesenchymal stromal cells; regenerative medicine; stromal vascular fraction.
Copyright © 2023 Cicione, Vadalà, Di Giacomo, Tilotta, Ambrosio, Russo, Zampogna, Cannata, Papalia and Denaro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bourin P., Bunnell B. A., Casteilla L., Dominici M., Katz A. J., March K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT). Cytotherapy 15 (6), 641–648. 10.1016/j.jcyt.2013.02.006 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

