Mitochondrial dysfunction in human hypertrophic cardiomyopathy is linked to cardiomyocyte architecture disruption and corrected by improving NADH-driven mitochondrial respiration
- PMID: 36734059
- PMCID: PMC10067466
- DOI: 10.1093/eurheartj/ehad028
Mitochondrial dysfunction in human hypertrophic cardiomyopathy is linked to cardiomyocyte architecture disruption and corrected by improving NADH-driven mitochondrial respiration
Abstract
Aims: Genetic hypertrophic cardiomyopathy (HCM) is caused by mutations in sarcomere protein-encoding genes (i.e. genotype-positive HCM). In an increasing number of patients, HCM occurs in the absence of a mutation (i.e. genotype-negative HCM). Mitochondrial dysfunction is thought to be a key driver of pathological remodelling in HCM. Reports of mitochondrial respiratory function and specific disease-modifying treatment options in patients with HCM are scarce.
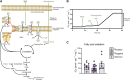
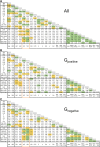
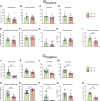
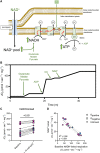
Methods and results: Respirometry was performed on septal myectomy tissue from patients with HCM (n = 59) to evaluate oxidative phosphorylation and fatty acid oxidation. Mitochondrial dysfunction was most notably reflected by impaired NADH-linked respiration. In genotype-negative patients, but not genotype-positive patients, NADH-linked respiration was markedly depressed in patients with an indexed septal thickness ≥10 compared with <10. Mitochondrial dysfunction was not explained by reduced abundance or fragmentation of mitochondria, as evaluated by transmission electron microscopy. Rather, improper organization of mitochondria relative to myofibrils (expressed as a percentage of disorganized mitochondria) was strongly associated with mitochondrial dysfunction. Pre-incubation with the cardiolipin-stabilizing drug elamipretide and raising mitochondrial NAD+ levels both boosted NADH-linked respiration.
Conclusion: Mitochondrial dysfunction is explained by cardiomyocyte architecture disruption and is linked to septal hypertrophy in genotype-negative HCM. Despite severe myocardial remodelling mitochondria were responsive to treatments aimed at restoring respiratory function, eliciting the mitochondria as a drug target to prevent and ameliorate cardiac disease in HCM. Mitochondria-targeting therapy may particularly benefit genotype-negative patients with HCM, given the tight link between mitochondrial impairment and septal thickening in this subpopulation.
Keywords: Cardiomyocyte architecture; Hypertrophic cardiomyopathy; Metabolism; Mitochondrial dysfunction; Mitochondrial therapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
K.B.M. is a consultant for Bristol Myers Squibb and receives research support from Amgen.
Figures





Comment in
-
Targeting mitochondria in hypertrophic cardiomyopathy.Eur Heart J. 2023 Apr 1;44(13):1186-1188. doi: 10.1093/eurheartj/ehad081. Eur Heart J. 2023. PMID: 36869826 No abstract available.
References
-
- Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. . 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020;76:e159–e240. 10.1016/j.jacc.2020.08.045 - DOI - PubMed

