Mild hypothermia attenuates ischaemia/reperfusion injury: insights from serial non-invasive pressure-volume loops
- PMID: 36734080
- PMCID: PMC10578916
- DOI: 10.1093/cvr/cvad028
Mild hypothermia attenuates ischaemia/reperfusion injury: insights from serial non-invasive pressure-volume loops
Abstract
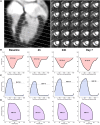
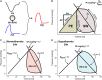
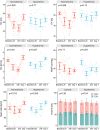
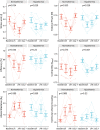
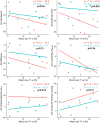
Aims: Mild hypothermia, 32-35°C, reduces infarct size in experimental studies, potentially mediating reperfusion injuries, but human trials have been ambiguous. To elucidate the cardioprotective mechanisms of mild hypothermia, we analysed cardiac performance in a porcine model of ischaemia/reperfusion, with serial cardiovascular magnetic resonance (CMR) imaging throughout 1 week using non-invasive pressure-volume (PV) loops.
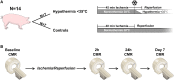
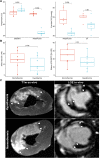
Methods and results: Normothermia and Hypothermia group sessions (n = 7 + 7 pigs, non-random allocation) were imaged with Cardiovascular magnetic resonance (CMR) at baseline and subjected to 40 min of normothermic ischaemia by catheter intervention. Thereafter, the Hypothermia group was rapidly cooled (mean 34.5°C) for 5 min before reperfusion. Additional CMR sessions at 2 h, 24 h, and 7 days acquired ventricular volumes and ischaemic injuries (unblinded analysis). Stroke volume (SV: -24%; P = 0.029; Friedmans test) and ejection fraction (EF: -20%; P = 0.068) were notably reduced at 24 h in the Normothermia group compared with baseline. In contrast, the decreases were ameliorated in the Hypothermia group (SV: -6%; P = 0.77; EF: -6%; P = 0.13). Mean arterial pressure remained stable in Normothermic animals (-3%, P = 0.77) but dropped 2 h post-reperfusion in hypothermic animals (-18%, P = 0.007). Both groups experienced a decrease and partial recovery pattern for PV loop-derived variables over 1 week, but the adverse effects tended to attenuate in the Hypothermia group. Infarct sizes were 10 ± 8% in Hypothermic and 15 ± 8% in Normothermic animals (P = 0.32). Analysis of covariance at 24 h indicated that hypothermia has cardioprotective properties incremental to reducing infarct size, such as higher external power (P = 0.061) and lower arterial elastance (P = 0.015).
Conclusion: Using non-invasive PV loops by CMR, we observed that mild hypothermia at reperfusion alleviates the heart's work after ischaemia/reperfusion injuries during the first week and preserves short-term cardiac performance. This hypothesis-generating study suggests hypothermia to have cardioprotective properties, incremental to reducing infarct size. The primary cardioprotective mechanism was likely an afterload reduction acutely unloading the left ventricle.
Keywords: Acute myocardial infarction; Hypothermia; Ischaemia; Pressure–volume loops; Reperfusion injury; induced.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: J.B. is an employee of Syntach AB, Lund, Sweden; H.A. is a stockholder in Imacor AB, Lund, Sweden; M.C. has received consultancy fees from Imacor AB for cardiac MRI analyses; E.H. is the owner and founder of Medviso AB, Lund, Sweden. Data can be made available upon reasonable request.
Figures

References
-
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao DW, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492. - PubMed
-
- Götberg M, Van Der PJ, Götberg M, Olivecrona GK, Kanski M, Koul S, Otto A, Engblom H, Ugander M, Arheden H, Erlinge D. Optimal timing of hypothermia in relation to myocardial reperfusion. Basic Res Cardiol 2011;106:697–708. - PubMed
-
- Erlinge D, Götberg M, Lang I, Holzer M, Noc M, Clemmensen P, Jensen U, Metzler B, James S, Bötker HE, Omerovic E, Engblom H, Carlsson M, Arheden H, Östlund O, Wallentin L, Harnek J, Olivecrona GK. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction: the CHILL-MI trial: a randomized controlled study of the use of central venous catheter. J Am Coll Cardiol 2014;63:1857–1865. - PubMed
-
- Götberg M, Olivecrona GK, Koul S, Carlsson M, Engblom H, Ugander M, Van Der PJ, Algotsson L, Arheden H, Erlinge D. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv 2010;3:400–407. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

