Efficacy and Safety of Fezolinetant in Moderate to Severe Vasomotor Symptoms Associated With Menopause: A Phase 3 RCT
- PMID: 36734148
- PMCID: PMC10348473
- DOI: 10.1210/clinem/dgad058
Efficacy and Safety of Fezolinetant in Moderate to Severe Vasomotor Symptoms Associated With Menopause: A Phase 3 RCT
Abstract
Context: Vasomotor symptoms (VMS) are common, bothersome, and can persist for years before and after menopause.
Objective: We aimed to assess efficacy/safety of fezolinetant for treatment of moderate to severe VMS associated with menopause.
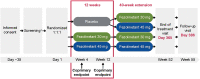
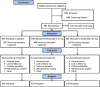
Methods: In this double-blind, placebo-controlled, 12-week phase 3 trial with a 40-week active treatment extension (NCT04003142; SKYLIGHT 2), women aged 40 to 65 years with minimum average 7 moderate to severe VMS/day were randomized to 12 weeks of once-daily placebo, fezolinetant 30 mg, or fezolinetant 45 mg. Completers were rerandomized to fezolinetant 30/45 mg for 40 additional weeks. Coprimary efficacy endpoints were mean daily change from baseline to week 4 (W4) and W12 in VMS frequency and severity. Safety was also assessed.
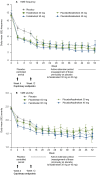
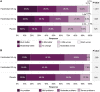
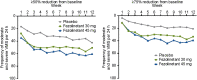
Results: Both fezolinetant doses statistically significantly reduced VMS frequency/severity at W4 and W12 vs placebo. For VMS frequency, W4 least squares mean (SE) reduction vs placebo: fezolinetant 30 mg, -1.82 (0.46; P < .001); 45 mg, -2.55 (0.46; P < .001); W12: 30 mg, -1.86 (0.55; P < .001); 45 mg, -2.53 (0.55; P < .001). For VMS severity, W4: 30 mg, -0.15 (0.06; P < .05); 45 mg, -0.29 (0.06; P < .001); W12: 30 mg, -0.16 (0.08; P < .05); 45 mg, -0.29 (0.08; P < .001). Improvement in VMS frequency and severity was observed by W1 and maintained through W52. Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo, respectively.
Conclusion: Daily fezolinetant 30 and 45 mg were efficacious and well tolerated for treating moderate to severe VMS associated with menopause.
Keywords: KNDy; fezolinetant; neurokinin 3 receptor antagonist; nonhormonal; vasomotor symptoms.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
Comment in
-
Neurokinin Receptor Antagonist, Fezolinetant, for Treatment of Menopausal Vasomotor Symptoms.J Clin Endocrinol Metab. 2023 Oct 18;108(11):e1448-e1449. doi: 10.1210/clinem/dgad209. J Clin Endocrinol Metab. 2023. PMID: 37097747 Free PMC article. No abstract available.
References
-
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211‐227. - PMC - PubMed

