Adult intracranial ependymoma-relevance of DNA methylation profiling for diagnosis, prognosis, and treatment
- PMID: 36734226
- PMCID: PMC10326475
- DOI: 10.1093/neuonc/noad030
Adult intracranial ependymoma-relevance of DNA methylation profiling for diagnosis, prognosis, and treatment
Abstract
Background: A methylation-based classification of ependymoma has recently found broad application. However, the diagnostic advantage and implications for treatment decisions remain unclear. Here, we retrospectively evaluate the impact of surgery and radiotherapy on outcome after molecular reclassification of adult intracranial ependymomas.
Methods: Tumors diagnosed as intracranial ependymomas from 170 adult patients collected from 8 diagnostic institutions were subjected to DNA methylation profiling. Molecular classes, patient characteristics, and treatment were correlated with progression-free survival (PFS).
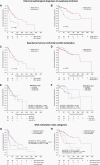
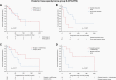
Results: The classifier indicated an ependymal tumor in 73.5%, a different tumor entity in 10.6%, and non-classifiable tumors in 15.9% of cases, respectively. The most prevalent molecular classes were posterior fossa ependymoma group B (EPN-PFB, 32.9%), posterior fossa subependymoma (PF-SE, 25.9%), and supratentorial ZFTA fusion-positive ependymoma (EPN-ZFTA, 11.2%). With a median follow-up of 60.0 months, the 5- and 10-year-PFS rates were 64.5% and 41.8% for EPN-PFB, 67.4% and 45.2% for PF-SE, and 60.3% and 60.3% for EPN-ZFTA. In EPN-PFB, but not in other molecular classes, gross total resection (GTR) (P = .009) and postoperative radiotherapy (P = .007) were significantly associated with improved PFS in multivariable analysis. Histological tumor grading (WHO 2 vs. 3) was not a predictor of the prognosis within molecularly defined ependymoma classes.
Conclusions: DNA methylation profiling improves diagnostic accuracy and risk stratification in adult intracranial ependymoma. The molecular class of PF-SE is unexpectedly prevalent among adult tumors with ependymoma histology and relapsed as frequently as EPN-PFB, despite the supposed benign nature. GTR and radiotherapy may represent key factors in determining the outcome of EPN-PFB patients.
Keywords: DNA methylation; adult; ependymoma; intracranial; radiotherapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

