Pretherapy Ferumoxytol-enhanced MRI to Predict Response to Liposomal Irinotecan in Metastatic Breast Cancer
- PMID: 36734848
- PMCID: PMC10077095
- DOI: 10.1148/rycan.220022
Pretherapy Ferumoxytol-enhanced MRI to Predict Response to Liposomal Irinotecan in Metastatic Breast Cancer
Abstract
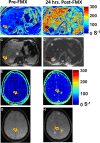
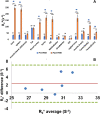
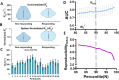
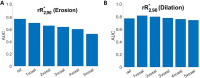
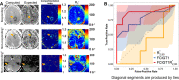
Purpose To investigate ferumoxytol (FMX)-enhanced MRI as a pretreatment predictor of response to liposomal irinotecan (nal-IRI) for thoracoabdominal and brain metastases in women with metastatic breast cancer (mBC). Materials and Methods In this phase 1 expansion trial (ClinicalTrials.gov identifier, NCT01770353; 27 participants), 49 thoracoabdominal (19 participants; mean age, 48 years ± 11 [SD]) and 19 brain (seven participants; mean age, 54 years ± 8) metastases were analyzed on MR images acquired before, 1-4 hours after, and 16-24 hours after FMX administration. In thoracoabdominal metastases, tumor transverse relaxation rate (R*2) was normalized to the mean R*2 in the spleen (rR*2), and the tumor histogram metric rR*2,N, representing the average of rR*2 in voxels above the nth percentile, was computed. In brain metastases, a novel compartmentation index was derived by applying the MRI signal equation to phantom-calibrated coregistered FMX-enhanced MRI brain scans acquired before, 1-4 hours after, and 16-24 hours after FMX administration. The fraction of voxels with an FMX compartmentation index greater than 1 was computed over the whole tumor (FCIGT1) and from voxels above the 90th percentile R*2 (FCIGT1 R*2,90). Results rR*2,90 computed from pretherapy MRI performed 16-24 hours after FMX administration, without reference to calibration phantoms, predicted response to nal-IRI in thoracoabdominal metastases (accuracy, 74%). rR*2,90 performance was robust to the inclusion of some peritumoral tissue within the tumor region of interest. FCIGT1 R*2,90 provided 79% accuracy on cross-validation in prediction of response in brain metastases. Conclusion This first in-human study focused on mBC suggests that FMX-enhanced MRI biologic markers can be useful for pretherapy prediction of response to nal-IRI in patients with mBC. Keywords: MRI Contrast Agent, MRI, Breast, Head/Neck, Tumor Response, Experimental Investigations, Brain/Brain Stem Clinical trial registration no. NCT01770353 Supplemental material is available for this article. © RSNA, 2023 See also commentary by Daldrup-Link in this issue.
Keywords: Brain/Brain Stem; Breast; Experimental Investigations; Head/Neck; MRI; MRI Contrast Agent; Tumor Response.
Conflict of interest statement
Figures




Comment in
-
Pretherapy Ferumoxytol-enhanced MRI for Metastatic Breast Cancer: A New Approach for Predicting Tumor Delivery of Macromolecular Therapeutics?Radiol Imaging Cancer. 2023 Mar;5(2):e220183. doi: 10.1148/rycan.220183. Radiol Imaging Cancer. 2023. PMID: 36734849 Free PMC article. No abstract available.
References
-
- Sung H , Ferlay J , Siegel RL , et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries . CA Cancer J Clin 2021. ; 71 ( 3 ): 209 – 249 . - PubMed
-
- Siegel RL , Miller KD , Jemal A . Cancer statistics, 2016 . CA Cancer J Clin 2016. ; 66 ( 1 ): 7 – 30 . - PubMed
-
- Shigeoka Y , Itoh K , Igarashi T , et al . Clinical effect of irinotecan in advanced and metastatic breast cancer patients previously treated with doxorubicin- and docetaxel-containing regimens . Jpn J Clin Oncol 2001. ; 31 ( 8 ): 370 – 374 . - PubMed
-
- Noble CO , Krauze MT , Drummond DC , et al . Pharmacokinetics, tumor accumulation and antitumor activity of nanoliposomal irinotecan following systemic treatment of intracranial tumors . Nanomedicine (Lond) 2014. ; 9 ( 14 ): 2099 – 2108 . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

