Structure-Activity Relationship of N-Ethyl-Hexedrone Analogues: Role of the α-Carbon Side-Chain Length in the Mechanism of Action, Cytotoxicity, and Behavioral Effects in Mice
- PMID: 36734852
- PMCID: PMC9936538
- DOI: 10.1021/acschemneuro.2c00772
Structure-Activity Relationship of N-Ethyl-Hexedrone Analogues: Role of the α-Carbon Side-Chain Length in the Mechanism of Action, Cytotoxicity, and Behavioral Effects in Mice
Abstract
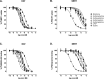
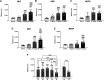
Synthetic cathinones are β-keto amphetamine derivatives whose appearance has increased dramatically in the past decades. N-Ethyl substituted cathinones have been proven to potently inhibit dopamine (DA) uptake and induce psychostimulant and rewarding effects in mice. However, little is known about the influence of the alpha-carbon side-chain length of N-ethyl cathinones on their pharmacological and toxicological effects. Thus, the aim of this study was to synthesize and investigate the in vitro and in vivo effects of five N-ethyl substituted cathinones: N-ethyl-cathinone (NEC), N-ethyl-buphedrone (NEB), N-ethyl-pentedrone, N-ethyl-hexedrone (NEH), and N-ethyl-heptedrone. HEK293 cells expressing the human DA or serotonin transporter (hDAT and hSERT) were used for uptake inhibition and binding assays. PC12 cells were used for the cytotoxicity assays. Swiss CD-1 mice were used to study the in vivo psychostimulant, anxiogenic, and rewarding properties. Our results show that all tested cathinones are able to inhibit DA uptake and are DAT-selective. The potency of DA uptake inhibitors increases with the elongation of the aliphatic side chain from methyl to propyl and decreases when increasing from butyl to pentyl, which correlates with an inverted U-shape psychostimulant response in mice at the medium dose tested. On the other hand, an increase in the α-carbon side-chain length correlates with an increase in the cytotoxic properties in PC12 cells, probably due to better membrane penetration. Moreover, all the cathinones tested have shown higher cytotoxicity than methamphetamine. Finally, our study not only demonstrated the rewarding properties of NEC and NEB but also the anxiety-like behavior induced at high doses by all the cathinones tested.
Keywords: anxiety; cytotoxicity; new psychoactive substances; psychostimulant; reward; synthetic cathinones.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





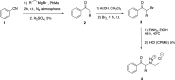
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

